Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19
- PMID: 32926097
- PMCID: PMC7488867
- DOI: 10.1084/jem.20201012
Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19
Abstract
Infection with SARS-CoV-2 is causing a deadly and pandemic disease called coronavirus disease-19 (COVID-19). While SARS-CoV-2-triggered hyperinflammatory tissue-damaging and immunothrombotic responses are thought to be major causes of respiratory failure and death, how they relate to lung immunopathological changes remains unclear. Neutrophil extracellular traps (NETs) can contribute to inflammation-associated lung damage, thrombosis, and fibrosis. However, whether NETs infiltrate particular compartments in severe COVID-19 lungs remains to be clarified. Here we analyzed postmortem lung specimens from four patients who succumbed to COVID-19 and four patients who died from a COVID-19-unrelated cause. We report the presence of NETs in the lungs of each COVID-19 patient. NETs were found in the airway compartment and neutrophil-rich inflammatory areas of the interstitium, while NET-prone primed neutrophils were present in arteriolar microthrombi. Our results support the hypothesis that NETs may represent drivers of severe pulmonary complications of COVID-19 and suggest that NET-targeting approaches could be considered for the treatment of uncontrolled tissue-damaging and thrombotic responses in COVID-19.
© 2020 Radermecker et al.
Conflict of interest statement
Disclosures: E. Cavalier reported "other" from Diasorin, Fujirebio, BioMerieux, IDS, Menarini, and Nittobo outside the submitted work. No other disclosures were reported.
Figures
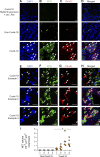

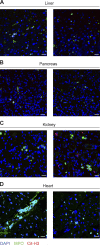


Similar articles
-
SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology.J Exp Med. 2020 Dec 7;217(12):e20201129. doi: 10.1084/jem.20201129. J Exp Med. 2020. PMID: 32926098 Free PMC article.
-
Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19.Cells. 2020 Jun 2;9(6):1383. doi: 10.3390/cells9061383. Cells. 2020. PMID: 32498376 Free PMC article.
-
SARS-CoV2 may evade innate immune response, causing uncontrolled neutrophil extracellular traps formation and multi-organ failure.Clin Sci (Lond). 2020 Jun 26;134(12):1295-1300. doi: 10.1042/CS20200531. Clin Sci (Lond). 2020. PMID: 32543703
-
Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression.Adv Biol Regul. 2020 Aug;77:100741. doi: 10.1016/j.jbior.2020.100741. Epub 2020 Jul 4. Adv Biol Regul. 2020. PMID: 32773102 Free PMC article. Review.
-
Early Insights into Immune Responses during COVID-19.J Immunol. 2020 Aug 1;205(3):555-564. doi: 10.4049/jimmunol.2000526. Epub 2020 Jun 8. J Immunol. 2020. PMID: 32513850 Review.
Cited by
-
Arterial Thrombosis in Acute Respiratory Infections: An Underestimated but Clinically Relevant Problem.J Clin Med. 2024 Oct 9;13(19):6007. doi: 10.3390/jcm13196007. J Clin Med. 2024. PMID: 39408067 Free PMC article. Review.
-
Characterization of Neutrophil Functional Responses to SARS-CoV-2 Infection in a Translational Feline Model for COVID-19.Int J Mol Sci. 2024 Sep 19;25(18):10054. doi: 10.3390/ijms251810054. Int J Mol Sci. 2024. PMID: 39337543 Free PMC article.
-
Emergence of dysfunctional neutrophils with a defect in arginase-1 release in severe COVID-19.JCI Insight. 2024 Sep 10;9(17):e171659. doi: 10.1172/jci.insight.171659. JCI Insight. 2024. PMID: 39253969 Free PMC article.
-
The assembly of neutrophil inflammasomes during COVID-19 is mediated by type I interferons.PLoS Pathog. 2024 Aug 22;20(8):e1012368. doi: 10.1371/journal.ppat.1012368. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39172744 Free PMC article.
-
Compartmentalization of the inflammatory response during bacterial sepsis and severe COVID-19.J Intensive Med. 2024 Feb 27;4(3):326-340. doi: 10.1016/j.jointm.2024.01.001. eCollection 2024 Jul. J Intensive Med. 2024. PMID: 39035623 Free PMC article. Review.
References
-
- Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., Palmer O.R., Bockenstedt P.L., Pinsky D.J., Greve J.M., et al. . 2019. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 10:1916 10.1038/s41467-019-09801-x - DOI - PMC - PubMed
-
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. . 2020. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 217 e20200652 10.1084/jem.20200652 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

