Pericytes in the infarcted heart
- PMID: 32923950
- PMCID: PMC7439839
- DOI: 10.1530/VB-19-0007
Pericytes in the infarcted heart
Abstract
The adult mammalian heart lacks regenerative capacity and heals through activation of an inflammatory cascade that leads to the formation of a collagen-based scar. Although scar formation is important to preserve the structural integrity of the ventricle, unrestrained inflammation and excessive fibrosis have been implicated in the pathogenesis of adverse post-infarction remodeling and heart failure. Interstitial cells play a crucial role in the regulation of cardiac repair. Although recent studies have explored the role of fibroblasts and immune cells, the cardiac pericytes have been largely ignored by investigators interested in myocardial biology. This review manuscript discusses the role of pericytes in the regulation of inflammation, fibrosis and angiogenesis following myocardial infarction. During the inflammatory phase of infarct healing, pericytes may regulate microvascular permeability and may play an important role in leukocyte trafficking. Moreover, pericyte activation through Toll-like receptor-mediated pathways may stimulate cytokine and chemokine synthesis. During the proliferative phase, pericytes may be involved in angiogenesis and fibrosis. To what extent pericyte to fibroblast conversion and pericyte-mediated growth factor synthesis contribute to the myocardial fibrotic response remains unknown. During the maturation phase of infarct healing, coating of infarct neovessels with pericytes plays an important role in scar stabilization. Implementation of therapeutic approaches targeting pericytes in the infarcted and remodeling heart remains challenging, due to the lack of systematic characterization of myocardial pericytes, their phenotypic heterogeneity and the limited knowledge on their functional role.
Keywords: angiogenesis; fibrosis; inflammation; myocardial infarction; pericyte.
© 2019 The authors.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Figures
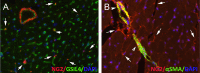

Similar articles
-
Cardiac Pericytes Acquire a Fibrogenic Phenotype and Contribute to Vascular Maturation After Myocardial Infarction.Circulation. 2023 Sep 12;148(11):882-898. doi: 10.1161/CIRCULATIONAHA.123.064155. Epub 2023 Jun 23. Circulation. 2023. PMID: 37350296 Free PMC article.
-
The fate and role of the pericytes in myocardial diseases.Eur J Clin Invest. 2024 Aug;54(8):e14204. doi: 10.1111/eci.14204. Epub 2024 Apr 8. Eur J Clin Invest. 2024. PMID: 38586936 Review.
-
Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction.Cells. 2022 Apr 20;11(9):1386. doi: 10.3390/cells11091386. Cells. 2022. PMID: 35563692 Free PMC article. Review.
-
Repair of the Infarcted Heart: Cellular Effectors, Molecular Mechanisms and Therapeutic Opportunities.Circ Res. 2024 Jun 7;134(12):1718-1751. doi: 10.1161/CIRCRESAHA.124.323658. Epub 2024 Jun 6. Circ Res. 2024. PMID: 38843294 Review.
-
The immune system and cardiac repair.Pharmacol Res. 2008 Aug;58(2):88-111. doi: 10.1016/j.phrs.2008.06.007. Epub 2008 Jun 24. Pharmacol Res. 2008. PMID: 18620057 Free PMC article. Review.
Cited by
-
Stromal Cell-SLIT3/Cardiomyocyte-ROBO1 Axis Regulates Pressure Overload-Induced Cardiac Hypertrophy.Circ Res. 2024 Mar 29;134(7):913-930. doi: 10.1161/CIRCRESAHA.122.321292. Epub 2024 Feb 27. Circ Res. 2024. PMID: 38414132 Free PMC article.
-
Mapping the cardiac vascular niche in heart failure.Nat Commun. 2022 May 31;13(1):3027. doi: 10.1038/s41467-022-30682-0. Nat Commun. 2022. PMID: 35641541 Free PMC article.
-
The neovascularization effect of dedifferentiated fat cells.Sci Rep. 2020 Jun 8;10(1):9211. doi: 10.1038/s41598-020-66135-1. Sci Rep. 2020. PMID: 32514018 Free PMC article.
-
Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control.Theranostics. 2021 May 3;11(14):6766-6785. doi: 10.7150/thno.60143. eCollection 2021. Theranostics. 2021. PMID: 34093852 Free PMC article. Review.
-
Resident stem cells in the heart.Med Rev (2021). 2021 Oct 21;1(1):10-13. doi: 10.1515/mr-2021-0003. eCollection 2021 Oct. Med Rev (2021). 2021. PMID: 37724080 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

