Under construction: The dynamic assembly, maintenance, and degradation of the cardiac sarcomere
- PMID: 32920010
- PMCID: PMC7736463
- DOI: 10.1016/j.yjmcc.2020.08.018
Under construction: The dynamic assembly, maintenance, and degradation of the cardiac sarcomere
Abstract
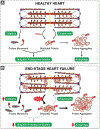
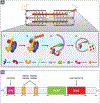
The sarcomere is the basic contractile unit of striated muscle and is a highly ordered protein complex with the actin and myosin filaments at its core. Assembling the sarcomere constituents into this organized structure in development, and with muscle growth as new sarcomeres are built, is a complex process coordinated by numerous factors. Once assembled, the sarcomere requires constant maintenance as its continuous contraction is accompanied by elevated mechanical, thermal, and oxidative stress, which predispose proteins to misfolding and toxic aggregation. To prevent protein misfolding and maintain sarcomere integrity, the sarcomere is monitored by an assortment of protein quality control (PQC) mechanisms. The need for effective PQC is heightened in cardiomyocytes which are terminally differentiated and must survive for many years while preserving optimal mechanical output. To prevent toxic protein aggregation, molecular chaperones stabilize denatured sarcomere proteins and promote their refolding. However, when old and misfolded proteins cannot be salvaged by chaperones, they must be recycled via degradation pathways: the calpain and ubiquitin-proteasome systems, which operate under basal conditions, and the stress-responsive autophagy-lysosome pathway. Mutations to and deficiency of the molecular chaperones and associated factors charged with sarcomere maintenance commonly lead to sarcomere structural disarray and the progression of heart disease, highlighting the necessity of effective sarcomere PQC for maintaining cardiac function. This review focuses on the dynamic regulation of assembly and turnover at the sarcomere with an emphasis on the chaperones involved in these processes and describes the alterations to chaperones - through mutations and deficient expression - implicated in disease progression to heart failure.
Keywords: Autophagy; Calpain; Chaperone; Proteasome; Protein quality control; Sarcomere; Sarcomerogenesis; Ubiquitin.
Copyright © 2020. Published by Elsevier Ltd.
Figures

Similar articles
-
Build it up-Tear it down: protein quality control in the cardiac sarcomere.Cardiovasc Res. 2009 Feb 15;81(3):439-48. doi: 10.1093/cvr/cvn289. Epub 2008 Oct 29. Cardiovasc Res. 2009. PMID: 18974044 Free PMC article. Review.
-
Chaperones and the Proteasome System: Regulating the Construction and Demolition of Striated Muscle.Int J Mol Sci. 2017 Dec 22;19(1):32. doi: 10.3390/ijms19010032. Int J Mol Sci. 2017. PMID: 29271938 Free PMC article. Review.
-
Early incorporation of obscurin into nascent sarcomeres: implication for myofibril assembly during cardiac myogenesis.Histochem Cell Biol. 2008 Apr;129(4):463-78. doi: 10.1007/s00418-008-0378-y. Epub 2008 Jan 25. Histochem Cell Biol. 2008. PMID: 18219491 Free PMC article.
-
SUMO system - a key regulator in sarcomere organization.FEBS J. 2020 Jun;287(11):2176-2190. doi: 10.1111/febs.15263. Epub 2020 Mar 13. FEBS J. 2020. PMID: 32096922 Review.
-
Protein Quality Control by Molecular Chaperones in Neurodegeneration.Front Neurosci. 2017 Apr 6;11:185. doi: 10.3389/fnins.2017.00185. eCollection 2017. Front Neurosci. 2017. PMID: 28428740 Free PMC article. Review.
Cited by
-
Novel insights into sarcomere regulatory systems control of cardiac thin filament activation.J Gen Physiol. 2021 Jul 5;153(7):e202012777. doi: 10.1085/jgp.202012777. J Gen Physiol. 2021. PMID: 33740037 Free PMC article.
-
A transcriptional enhancer regulates cardiac maturation.Nat Cardiovasc Res. 2024 Jun;3(6):666-684. doi: 10.1038/s44161-024-00484-2. Epub 2024 May 30. Nat Cardiovasc Res. 2024. PMID: 39196225
-
Assessment of Autophagy Markers Suggests Increased Activity Following LVAD Therapy.JACC Basic Transl Sci. 2023 Aug 23;8(9):1043-1056. doi: 10.1016/j.jacbts.2023.05.015. eCollection 2023 Sep. JACC Basic Transl Sci. 2023. PMID: 37791310 Free PMC article.
-
Pharmacological inhibition of BAG3-HSP70 with the proposed cancer therapeutic JG-98 is toxic for cardiomyocytes.J Cell Biochem. 2022 Jan;123(1):128-141. doi: 10.1002/jcb.30140. Epub 2021 Sep 6. J Cell Biochem. 2022. PMID: 34487557 Free PMC article.
-
Turn(over) the Page: Advancing Understanding of Proteome Dynamics After Heart Attack.JACC Basic Transl Sci. 2024 Jun 24;9(6):808-810. doi: 10.1016/j.jacbts.2024.05.002. eCollection 2024 Jun. JACC Basic Transl Sci. 2024. PMID: 39070271 Free PMC article.
References
-
- Rollett A, Examinations on the construction of striated muscle, Austrian Acad. Sci (1884).
-
- Lewis YE, Moskovitz A, Mutlak M, Heineke J, Caspi LH, Kehat I, Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance, J. Mol. Cell. Cardiol 116 (2018) 16–28. - PubMed
-
- Ghosh SR, Hope IA, Determination of the mobility of novel and established Caenorhabditis elegans sarcomeric proteins in vivo, Eur. J. Cell Biol 89 (2010) 437–448. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

