Immune response in COVID-19: What do we currently know?
- PMID: 32916246
- PMCID: PMC7480770
- DOI: 10.1016/j.micpath.2020.104484
Immune response in COVID-19: What do we currently know?
Abstract
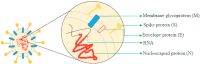
In 2002/2003 there was a pandemic denominate SARS (severe acute respiratory syndrome), caused by the SARS-CoV virus that belongs to the genera Betacoranavirus and the family Coronaviridae, generally responsible for influenza infections. In mid of 2019, a new disease by the coronavirus named by COVID-19 (SARS-CoV-2) emerged, both infections have flu symptoms, however they are infections that variable intensity, being medium to severe. In medium infections individuals have the virus and exhibit symptoms, however hospitalization is not necessary, in severe infections, individuals are hospitalized, have high pathology and in some cases progress to death. The virus is formed by simple positive RNA, enveloped, non-segmented, and presenting the largest genome of viruses constituting 32 Kb, consisting of envelope proteins, membrane, nucleocapsid and spike protein, which is essential in the interaction with the host cells. As for the origin of this virus, research has been intensified to determine this paradox and although the similarity with SARS-CoV, this virus did not has necessarily the same place of origin. As for the immune system, it is currently unknown how this new virus interacts. In this brief review, we demonstrate important considerations about the responses to this infection.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors declare no commercial or financial conflict of interest.
Figures
Similar articles
-
Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants.Elife. 2020 Oct 28;9:e61312. doi: 10.7554/eLife.61312. Elife. 2020. PMID: 33112236 Free PMC article.
-
Kinetics of Nucleocapsid, Spike and Neutralizing Antibodies, and Viral Load in Patients with Severe COVID-19 Treated with Convalescent Plasma.Viruses. 2021 Sep 15;13(9):1844. doi: 10.3390/v13091844. Viruses. 2021. PMID: 34578426 Free PMC article. Clinical Trial.
-
Cross-reactivity of SARS-CoV structural protein antibodies against SARS-CoV-2.Cell Rep. 2021 Feb 16;34(7):108737. doi: 10.1016/j.celrep.2021.108737. Epub 2021 Jan 26. Cell Rep. 2021. PMID: 33545052 Free PMC article.
-
The SARS-CoV-2 Spike Glycoprotein as a Drug and Vaccine Target: Structural Insights into Its Complexes with ACE2 and Antibodies.Cells. 2020 Oct 22;9(11):2343. doi: 10.3390/cells9112343. Cells. 2020. PMID: 33105869 Free PMC article. Review.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
Cited by
-
Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications.Brain Sci. 2021 Dec 31;12(1):59. doi: 10.3390/brainsci12010059. Brain Sci. 2021. PMID: 35053804 Free PMC article. Review.
-
Association of triglyceride/high-density lipoprotein cholesterol ratio with severe complications of COVID-19.Heliyon. 2023 Jun;9(6):e17428. doi: 10.1016/j.heliyon.2023.e17428. Epub 2023 Jun 17. Heliyon. 2023. PMID: 37366523 Free PMC article.
-
Evaluation of the Levels of Peripheral CD3+, CD4+, and CD8+ T Cells and IgG and IgM Antibodies in COVID-19 Patients at Different Stages of Infection.Microbiol Spectr. 2022 Feb 23;10(1):e0084521. doi: 10.1128/spectrum.00845-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196808 Free PMC article.
-
Metabolomics Signatures of SARS-CoV-2 Infection.Adv Exp Med Biol. 2022;1376:45-59. doi: 10.1007/5584_2021_674. Adv Exp Med Biol. 2022. PMID: 34735713 Review.
-
Temporal patterns of cytokine and injury biomarkers in hospitalized COVID-19 patients treated with methylprednisolone.Front Immunol. 2023 Aug 16;14:1229611. doi: 10.3389/fimmu.2023.1229611. eCollection 2023. Front Immunol. 2023. PMID: 37662953 Free PMC article.
References
-
- WHO-World Health Organization Mers situation update. 2020. http://applications.emro.who.int/docs/Emrpub-Csr-241-2019-En.pdf?ua=1&ua... Available in:
-
- Chan K.H. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229e, Oc43, and Nl63. Clin. Diagn. Lab. Immunol. 2005;12(11):1317–1321. In .Gorse, G. J.; Donovan, M.M.; Patel, G. B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. Journal of Medical Virology, feb.2020. - PMC - PubMed
-
- Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4(3):26. jul.2016.In Gorse, G. J.; Donovan, M.M.; Patel, G. B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. Journal of Medical Virology, feb.2020. - PMC - PubMed
-
- Zhao J. Recovery from the Middle East respiratory syndrome is associated with antibody and T cell responses. Sci. Immunol. aug.2017;2(14) In. Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in immunopathology. Springer Berlin Heidelberg, pp. 529–539, feb. 2017. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous