Site-specific deacylation by ABHD17a controls BK channel splice variant activity
- PMID: 32913120
- PMCID: PMC7864050
- DOI: 10.1074/jbc.RA120.015349
Site-specific deacylation by ABHD17a controls BK channel splice variant activity
Abstract
S-Acylation, the reversible post-translational lipid modification of proteins, is an important mechanism to control the properties and function of ion channels and other polytopic transmembrane proteins. However, although increasing evidence reveals the role of diverse acyl protein transferases (zDHHC) in controlling ion channel S-acylation, the acyl protein thioesterases that control ion channel deacylation are very poorly defined. Here we show that ABHD17a (α/β-hydrolase domain-containing protein 17a) deacylates the stress-regulated exon domain of large conductance voltage- and calcium-activated potassium (BK) channels inhibiting channel activity independently of effects on channel surface expression. Importantly, ABHD17a deacylates BK channels in a site-specific manner because it has no effect on the S-acylated S0-S1 domain conserved in all BK channels that controls membrane trafficking and is deacylated by the acyl protein thioesterase Lypla1. Thus, distinct S-acylated domains in the same polytopic transmembrane protein can be regulated by different acyl protein thioesterases revealing mechanisms for generating both specificity and diversity for these important enzymes to control the properties and functions of ion channels.
Keywords: Kcnma1; Kcnmb1; S-acylation; acyl protein thioesterase; acyl thioesterase; ion channel; lipid; lipid modification; membrane trafficking; palmitoylation; post-translational modification (PTM); potassium channel; protein trafficking.
© 2020 McClafferty et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures

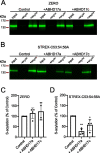
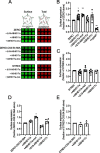
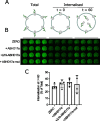
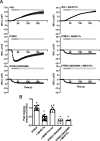

Comment in
-
Cutting out the fat: Site-specific deacylation of an ion channel.J Biol Chem. 2020 Dec 4;295(49):16497-16498. doi: 10.1074/jbc.H120.016490. J Biol Chem. 2020. PMID: 33277403 Free PMC article.
Similar articles
-
Cutting out the fat: Site-specific deacylation of an ion channel.J Biol Chem. 2020 Dec 4;295(49):16497-16498. doi: 10.1074/jbc.H120.016490. J Biol Chem. 2020. PMID: 33277403 Free PMC article.
-
S-Acylation controls functional coupling of BK channel pore-forming α-subunits and β1-subunits.J Biol Chem. 2019 Aug 9;294(32):12066-12076. doi: 10.1074/jbc.RA119.009065. Epub 2019 Jun 18. J Biol Chem. 2019. PMID: 31213527 Free PMC article.
-
Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels.J Biol Chem. 2012 Apr 27;287(18):14718-25. doi: 10.1074/jbc.M111.335547. Epub 2012 Mar 7. J Biol Chem. 2012. PMID: 22399288 Free PMC article.
-
S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels.Front Physiol. 2014 Aug 5;5:281. doi: 10.3389/fphys.2014.00281. eCollection 2014. Front Physiol. 2014. PMID: 25140154 Free PMC article. Review.
-
Posttranscriptional and Posttranslational Regulation of BK Channels.Int Rev Neurobiol. 2016;128:91-126. doi: 10.1016/bs.irn.2016.02.012. Epub 2016 Mar 3. Int Rev Neurobiol. 2016. PMID: 27238262 Review.
Cited by
-
Identification of biomarkers for immunotherapy response in prostate cancer and potential drugs to alleviate immunosuppression.Aging (Albany NY). 2022 Jun 8;14(11):4839-4857. doi: 10.18632/aging.204115. Epub 2022 Jun 8. Aging (Albany NY). 2022. PMID: 35680563 Free PMC article.
-
Activity-dependent post-translational regulation of palmitoylating and depalmitoylating enzymes in the hippocampus.J Cell Sci. 2023 Apr 1;136(7):jcs260629. doi: 10.1242/jcs.260629. Epub 2023 Apr 5. J Cell Sci. 2023. PMID: 37039765 Free PMC article.
-
Substrate recruitment by zDHHC protein acyltransferases.Open Biol. 2021 Apr;11(4):210026. doi: 10.1098/rsob.210026. Epub 2021 Apr 21. Open Biol. 2021. PMID: 33878949 Free PMC article.
-
Cutting out the fat: Site-specific deacylation of an ion channel.J Biol Chem. 2020 Dec 4;295(49):16497-16498. doi: 10.1074/jbc.H120.016490. J Biol Chem. 2020. PMID: 33277403 Free PMC article.
-
Refining S-acylation: Structure, regulation, dynamics, and therapeutic implications.J Cell Biol. 2023 Nov 6;222(11):e202307103. doi: 10.1083/jcb.202307103. Epub 2023 Sep 27. J Cell Biol. 2023. PMID: 37756661 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

