The Secreted Glycoprotein Reelin Suppresses the Proliferation and Regulates the Distribution of Oligodendrocyte Progenitor Cells in the Embryonic Neocortex
- PMID: 32913108
- PMCID: PMC7531546
- DOI: 10.1523/JNEUROSCI.0125-20.2020
The Secreted Glycoprotein Reelin Suppresses the Proliferation and Regulates the Distribution of Oligodendrocyte Progenitor Cells in the Embryonic Neocortex
Abstract
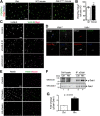
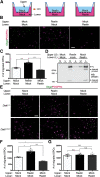
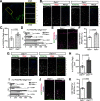
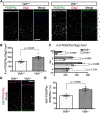
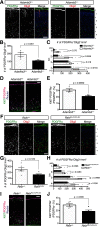
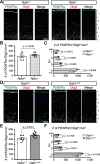
Oligodendrocyte (OL) progenitor cells (OPCs) are generated, proliferate, migrate, and differentiate in the developing brain. Although the development of OPCs is prerequisite for normal brain function, the molecular mechanisms regulating their development in the neocortex are not fully understood. Several molecules regulate the tangential distribution of OPCs in the developing neocortex, but the cue molecule(s) that regulate their radial distribution remains unknown. Here, we demonstrate that the secreted glycoprotein Reelin suppresses the proliferation of OPCs and acts as a repellent for their migration in vitro These functions rely on the binding of Reelin to its receptors and on the signal transduction involving the intracellular protein Dab1. In the late embryonic neocortex of mice with attenuated Reelin signaling [i.e., Reelin heterozygote-deficient, Dab1 heterozygote-deficient mutant, or very low-density lipoprotein receptor (VLDLR)-deficient mice], the number of OPCs increased and their distribution shifted toward the superficial layers. In contrast, the number of OPCs decreased and they tended to distribute in the deep layers in the neocortex of mice with abrogated inactivation of Reelin by proteolytic cleavage, namely a disintegrin and metalloproteinase with thrombospondin type 1 motifs 3 (ADAMTS-3)-deficient mice and cleavage-resistant Reelin knock-in mice. Both male and female animals were used. These data indicate that Reelin-Dab1 signaling regulates the proliferation and radial distribution of OPCs in the late embryonic neocortex and that the regulation of Reelin function by its specific proteolysis is required for the normal development of OPCs.SIGNIFICANCE STATEMENT Here, we report that Reelin-Dab1 signaling regulates the proliferation and radial distribution of OPCs in the late embryonic mouse neocortex. Oligodendrocyte (OL) progenitor cells (OPCs) express Reelin signaling molecules and respond to Reelin stimulation. Reelin-Dab1 signaling suppresses the proliferation of OPCs both in vitro and in vivo Reelin repels OPCs in vitro, and the radial distribution of OPCs is altered in mice with either attenuated or augmented Reelin-Dab1 signaling. This is the first report identifying the secreted molecule that plays a role in the radial distribution of OPCs in the late embryonic neocortex. Our results also show that the regulation of Reelin function by its specific proteolysis is important for the normal development of OPCs.
Keywords: Dab1; Reelin; migration; neocortex; oligodendrocyte progenitor cell.
Copyright © 2020 the authors.
Figures


Similar articles
-
Reelin-Nrp1 Interaction Regulates Neocortical Dendrite Development in a Context-Specific Manner.J Neurosci. 2020 Oct 21;40(43):8248-8261. doi: 10.1523/JNEUROSCI.1907-20.2020. Epub 2020 Oct 2. J Neurosci. 2020. PMID: 33009002 Free PMC article.
-
Secreted Metalloproteinase ADAMTS-3 Inactivates Reelin.J Neurosci. 2017 Mar 22;37(12):3181-3191. doi: 10.1523/JNEUROSCI.3632-16.2017. Epub 2017 Feb 17. J Neurosci. 2017. PMID: 28213441 Free PMC article.
-
Reelin promotes oligodendrocyte precursors migration via the Wnt/β-catenin signaling pathway.Neurol Res. 2021 Jul;43(7):543-552. doi: 10.1080/01616412.2021.1888604. Epub 2021 Feb 21. Neurol Res. 2021. PMID: 33616025
-
Strange bedfellows: Reelin and Notch signaling interact to regulate cell migration in the developing neocortex.Neuron. 2008 Oct 23;60(2):189-91. doi: 10.1016/j.neuron.2008.10.009. Neuron. 2008. PMID: 18957210 Review.
-
Evolution of cortical lamination: the reelin/Dab1 pathway.Novartis Found Symp. 2000;228:114-25; discussion 125-8. doi: 10.1002/0470846631.ch9. Novartis Found Symp. 2000. PMID: 10929319 Review.
Cited by
-
Reelin Signaling in Neurodevelopmental Disorders and Neurodegenerative Diseases.Brain Sci. 2023 Oct 19;13(10):1479. doi: 10.3390/brainsci13101479. Brain Sci. 2023. PMID: 37891846 Free PMC article. Review.
-
Non-electric bioelectrical analog strategy by a biophysical-driven nano-micro spatial anisotropic scaffold for regulating stem cell niche and tissue regeneration in a neuronal therapy.Bioact Mater. 2022 Jun 13;20:319-338. doi: 10.1016/j.bioactmat.2022.05.034. eCollection 2023 Feb. Bioact Mater. 2022. PMID: 36380746 Free PMC article.
-
The Inflammation-Induced Dysregulation of Reelin Homeostasis Hypothesis of Alzheimer's Disease.J Alzheimers Dis. 2024;100(4):1099-1119. doi: 10.3233/JAD-240088. J Alzheimers Dis. 2024. PMID: 38995785 Free PMC article. Review.
-
Considering the Role of Extracellular Matrix Molecules, in Particular Reelin, in Granule Cell Dispersion Related to Temporal Lobe Epilepsy.Front Cell Dev Biol. 2022 Jun 6;10:917575. doi: 10.3389/fcell.2022.917575. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35733853 Free PMC article. Review.
-
Developmental Cajal-Retzius cell death contributes to the maturation of layer 1 cortical inhibition and somatosensory processing.Nat Commun. 2024 Aug 1;15(1):6501. doi: 10.1038/s41467-024-50658-6. Nat Commun. 2024. PMID: 39090081 Free PMC article.
References
-
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
-
- Bradley A, Anastassiadis K, Ayadi A, Battey JF, Bell C, Birling MC, Bottomley J, Brown SD, Bürger A, Bult CJ, Bushell W, Collins FS, Desaintes C, Doe B, Economides A, Eppig JT, Finnell RH, Fletcher C, Fray M, Frendewey D, et al. (2012) The mammalian gene function resource: the international knockout mouse consortium. Mamm Genome 23:580–586. 10.1007/s00335-012-9422-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
