Extracellular Alpha-Synuclein Promotes a Neuroinhibitory Secretory Phenotype in Astrocytes
- PMID: 32911644
- PMCID: PMC7555668
- DOI: 10.3390/life10090183
Extracellular Alpha-Synuclein Promotes a Neuroinhibitory Secretory Phenotype in Astrocytes
Abstract
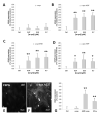
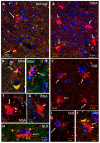
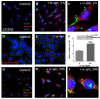
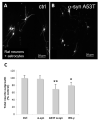
Multiple system atrophy (MSA) and dementia with Lewy bodies (DLB) are α-synucleinopathies that exhibit widespread astrogliosis as a component of the neuroinflammatory response. Munc18, a protein critical to vesicle exocytosis, was previously found to strongly mark morphologically activated astrocytes in brain tissue of MSA patients. Immunofluorescence of MSA, DLB and normal brain tissue sections was combined with cell culture and co-culture experiments to investigate the relationship between extracellular α-synuclein and the transition to a secretory astrocyte phenotype. Increased Munc18-positive vesicles were resolved in activated astrocytes in MSA and DLB tissue compared to controls, and they were also significantly upregulated in the human 1321N1 astrocytoma cell line upon treatment with α-synuclein, with parallel increases in GFAP expression and IL-6 secretion. In co-culture experiments, rat primary astrocytes pretreated with α-synuclein inhibited the growth of neurites of co-cultured primary rat neurons and upregulated chondroitin sulphate proteoglycan. Taken together, these results indicate that the secretory machinery is significantly upregulated in the astrocyte response to extracellular α-synuclein and may participate in the release of neuroinhibitory and proinflammatory factors in α-synucleinopathies.
Keywords: DLB; MSA; Munc18; astrocytes; astrogliosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Replication of multiple system atrophy prions in primary astrocyte cultures from transgenic mice expressing human α-synuclein.Acta Neuropathol Commun. 2019 May 20;7(1):81. doi: 10.1186/s40478-019-0703-9. Acta Neuropathol Commun. 2019. PMID: 31109379 Free PMC article.
-
Alpha-synuclein accumulates in Purkinje cells in Lewy body disease but not in multiple system atrophy.J Neuropathol Exp Neurol. 2003 Aug;62(8):812-9. doi: 10.1093/jnen/62.8.812. J Neuropathol Exp Neurol. 2003. PMID: 14503637
-
The degree of astrocyte activation in multiple system atrophy is inversely proportional to the distance to α-synuclein inclusions.Mol Cell Neurosci. 2015 Mar;65:68-81. doi: 10.1016/j.mcn.2015.02.015. Epub 2015 Feb 27. Mol Cell Neurosci. 2015. PMID: 25731829
-
[Clinical and pathological study on early diagnosis of Parkinson's disease and dementia with Lewy bodies].Rinsho Shinkeigaku. 2008 Jan;48(1):11-24. doi: 10.5692/clinicalneurol.48.11. Rinsho Shinkeigaku. 2008. PMID: 18386627 Review. Japanese.
-
Neuropathological spectrum of synucleinopathies.Mov Disord. 2003 Sep;18 Suppl 6:S2-12. doi: 10.1002/mds.10557. Mov Disord. 2003. PMID: 14502650 Review.
Cited by
-
Astrocytic modulation of neuronal signalling.Front Netw Physiol. 2023 Jun 1;3:1205544. doi: 10.3389/fnetp.2023.1205544. eCollection 2023. Front Netw Physiol. 2023. PMID: 37332623 Free PMC article. Review.
-
Regulated exocytosis from astrocytes: a matter of vesicles?Front Neurosci. 2024 May 10;18:1393165. doi: 10.3389/fnins.2024.1393165. eCollection 2024. Front Neurosci. 2024. PMID: 38800570 Free PMC article. No abstract available.
-
Ferritinophagy and α-Synuclein: Pharmacological Targeting of Autophagy to Restore Iron Regulation in Parkinson's Disease.Int J Mol Sci. 2022 Feb 21;23(4):2378. doi: 10.3390/ijms23042378. Int J Mol Sci. 2022. PMID: 35216492 Free PMC article. Review.
-
A functional role for alpha-synuclein in neuroimmune responses.J Neuroimmunol. 2023 Mar 15;376:578047. doi: 10.1016/j.jneuroim.2023.578047. Epub 2023 Feb 8. J Neuroimmunol. 2023. PMID: 36791583 Free PMC article. Review.
-
Parkinson's disease: Alterations in iron and redox biology as a key to unlock therapeutic strategies.Redox Biol. 2021 May;41:101896. doi: 10.1016/j.redox.2021.101896. Epub 2021 Feb 14. Redox Biol. 2021. PMID: 33799121 Free PMC article. Review.
References
-
- Radford R., Wong M., Pountney D.L. Handbook of Neurotoxicity. Springer; New York, NY, USA: 2014. Neurodegenerative Aspects of Multiple System Atrophy; pp. 2157–2180.
-
- Gazulla J., Berciano J., Peyronnet B., Manunta A., Gamé X., Fanciulli A., Wenning G., Babu D.S. Multiple-System Atrophy. N. Engl. J. Med. 2015;372:249–263. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

