Small ubiquitin-like modifier 2 (SUMO2) is critical for memory processes in mice
- PMID: 32910521
- PMCID: PMC7606628
- DOI: 10.1096/fj.202000850RR
Small ubiquitin-like modifier 2 (SUMO2) is critical for memory processes in mice
Abstract
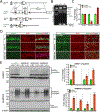
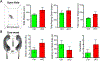
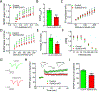
Small ubiquitin-like modifier (SUMO1-3) conjugation (SUMOylation), a posttranslational modification, modulates almost all major cellular processes. Mounting evidence indicates that SUMOylation plays a crucial role in maintaining and regulating neural function, and importantly its dysfunction is implicated in cognitive impairment in humans. We have previously shown that simultaneously silencing SUMO1-3 expression in neurons negatively affects cognitive function. However, the roles of the individual SUMOs in modulating cognition and the mechanisms that link SUMOylation to cognitive processes remain unknown. To address these questions, in this study, we have focused on SUMO2 and generated a new conditional Sumo2 knockout mouse line. We found that conditional deletion of Sumo2 predominantly in forebrain neurons resulted in marked impairments in various cognitive tests, including episodic and fear memory. Our data further suggest that these abnormalities are attributable neither to constitutive changes in gene expression nor to alterations in neuronal morphology, but they involve impairment in dynamic SUMOylation processes associated with synaptic plasticity. Finally, we provide evidence that dysfunction on hippocampal-based cognitive tasks was associated with a significant deficit in the maintenance of hippocampal long-term potentiation in Sumo2 knockout mice. Collectively, these data demonstrate that protein conjugation by SUMO2 is critically involved in cognitive processes.
Keywords: LTP; knockout; memory impairment; posttranslational modification.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of interest.
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Spatiotemporal distribution of SUMOylation components during mouse brain development.J Comp Neurol. 2014 Sep 1;522(13):3020-36. doi: 10.1002/cne.23563. J Comp Neurol. 2014. PMID: 24639124
-
SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development.EMBO Rep. 2014 Aug;15(8):878-85. doi: 10.15252/embr.201438534. Epub 2014 Jun 2. EMBO Rep. 2014. PMID: 24891386 Free PMC article.
-
Sumoylation differentially regulates Sp1 to control cell differentiation.Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5574-9. doi: 10.1073/pnas.1315034111. Epub 2014 Mar 27. Proc Natl Acad Sci U S A. 2014. PMID: 24706897 Free PMC article.
-
The post-translational modification, SUMOylation, and cancer (Review).Int J Oncol. 2018 Apr;52(4):1081-1094. doi: 10.3892/ijo.2018.4280. Epub 2018 Feb 22. Int J Oncol. 2018. PMID: 29484374 Free PMC article. Review.
-
SUMO2, a small ubiquitin-like modifier, is essential for development of murine preimplantation embryos.Theriogenology. 2021 May;166:29-37. doi: 10.1016/j.theriogenology.2021.01.019. Epub 2021 Feb 27. Theriogenology. 2021. PMID: 33677127 Review.
Cited by
-
SENP5 deteriorates traumatic brain injury via SUMO2-dependent suppression of E2F1 SUMOylation.Acta Biochim Biophys Sin (Shanghai). 2023 Jul 5;55(8):1193-1203. doi: 10.3724/abbs.2023121. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37403456 Free PMC article.
-
Sustained overexpression of spliced X-box-binding protein-1 in neurons leads to spontaneous seizures and sudden death in mice.Commun Biol. 2023 Mar 9;6(1):252. doi: 10.1038/s42003-023-04594-8. Commun Biol. 2023. PMID: 36894627 Free PMC article.
-
Paradoxes of Cellular SUMOylation Regulation: A Role of Biomolecular Condensates?Pharmacol Rev. 2023 Sep;75(5):979-1006. doi: 10.1124/pharmrev.122.000784. Epub 2023 May 3. Pharmacol Rev. 2023. PMID: 37137717 Free PMC article. Review.
-
Activation of the ATF6 (Activating Transcription Factor 6) Signaling Pathway in Neurons Improves Outcome After Cardiac Arrest in Mice.J Am Heart Assoc. 2021 Jun 15;10(12):e020216. doi: 10.1161/JAHA.120.020216. Epub 2021 Jun 11. J Am Heart Assoc. 2021. PMID: 34111943 Free PMC article.
-
SUMOylation Connects Cell Stress Responses and Inflammatory Control: Lessons From the Gut as a Model Organ.Front Immunol. 2021 Feb 19;12:646633. doi: 10.3389/fimmu.2021.646633. eCollection 2021. Front Immunol. 2021. PMID: 33679811 Free PMC article. Review.
References
-
- Ripamonti S, Shomroni O, Rhee JS, Chowdhury K, Jahn O, Hellmann KP, Bonn S, Brose N, and Tirard M (2020) SUMOylation controls the neurodevelopmental function of the transcription factor Zbtb20. J Neurochem. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

