High sensitivity of one-step real-time reverse transcription quantitative PCR to detect low virus titers in large mosquito pools
- PMID: 32907625
- PMCID: PMC7488135
- DOI: 10.1186/s13071-020-04327-4
High sensitivity of one-step real-time reverse transcription quantitative PCR to detect low virus titers in large mosquito pools
Abstract
Background: Mosquitoes are the deadliest animals in the world. Their ability to carry and spread diseases to humans causes millions of deaths every year. Due to the lack of efficient vaccines, the control of mosquito-borne diseases primarily relies on the management of the vector. Traditional control methods are insufficient to control mosquito populations. The sterile insect technique (SIT) is an additional control method that can be combined with other control tactics to suppress specific mosquito populations. The SIT requires the mass-rearing and release of sterile males with the aim to induce sterility in the wild female population. Samples collected from the environment for laboratory colonization, as well as the released males, should be free from mosquito-borne viruses (MBV). Therefore, efficient detection methods with defined detection limits for MBV are required. Although a one-step reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) method was developed to detect arboviruses in human and mosquito samples, its detection limit in mosquito samples has yet to be defined.
Methods: We evaluated the detection sensitivity of one step RT-qPCR for targeted arboviruses in large mosquito pools, using pools of non-infected mosquitoes of various sizes (165, 320 and 1600 mosquitoes) containing one infected mosquito body with defined virus titers of chikungunya virus (CHIKV), usutu virus (USUV), West Nile virus (WNV) and Zika virus (ZIKV).
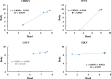
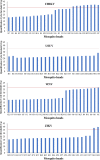
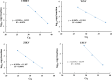
Results: CHIK, USUV, ZIKV, and WNV virus were detected in all tested pools using the RT-qPCR assay. Moreover, in the largest mosquito pools (1600 mosquitoes), RT-qPCR was able to detect the targeted viruses using different total RNA quantities (10, 1 and 0.1 ng per reaction) as a template. Correlating the virus titer with the total RNA quantity allowed the prediction of the maximum number of mosquitoes per pool in which the RT-qPCR can theoretically detect the virus infection.
Conclusions: Mosquito-borne viruses can be reliably detected by RT-qPCR assay in pools of mosquitoes exceeding 1000 specimens. This will represent an important step to expand pathogen-free colonies for mass-rearing sterile males for programmes that have a SIT component by reducing the time and the manpower needed to conduct this quality control process.
Keywords: Arbovirus; Chikungunya virus (CHIKV); Flavivirus; Pool size; RT-qPCR; Usutu virus (USUV); West Nile virus (WNV); Zika virus (ZIKV).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Mosquito-Borne Viruses and Insect-Specific Viruses Revealed in Field-Collected Mosquitoes by a Monitoring Tool Adapted from a Microbial Detection Array.Appl Environ Microbiol. 2019 Sep 17;85(19):e01202-19. doi: 10.1128/AEM.01202-19. Print 2019 Oct 1. Appl Environ Microbiol. 2019. PMID: 31350319 Free PMC article.
-
Arboviral surveillance of field-collected mosquitoes reveals circulation of West Nile virus lineage 1 strains in Eastern Thrace, Turkey.Vector Borne Zoonotic Dis. 2013 Oct;13(10):744-52. doi: 10.1089/vbz.2012.1288. Epub 2013 Aug 6. Vector Borne Zoonotic Dis. 2013. PMID: 23919608
-
Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009.PLoS One. 2010 Dec 15;5(12):e14324. doi: 10.1371/journal.pone.0014324. PLoS One. 2010. PMID: 21179462 Free PMC article.
-
Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy.Vet Ital. 2017 Jun 30;53(2):97-110. doi: 10.12834/VetIt.114.933.4764.2. Vet Ital. 2017. PMID: 28675249 Review.
-
Role of autophagy during the replication and pathogenesis of common mosquito-borne flavi- and alphaviruses.Open Biol. 2019 Mar 29;9(3):190009. doi: 10.1098/rsob.190009. Open Biol. 2019. PMID: 30862253 Free PMC article. Review.
Cited by
-
The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010-2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique.Insects. 2021 Apr 13;12(4):346. doi: 10.3390/insects12040346. Insects. 2021. PMID: 33924539 Free PMC article. Review.
-
A numbers game: Mosquito-based arbovirus surveillance in two distinct geographic regions of Latin America.bioRxiv [Preprint]. 2024 Mar 19:2024.03.15.585246. doi: 10.1101/2024.03.15.585246. bioRxiv. 2024. Update in: J Med Entomol. 2025 Jan 13;62(1):220-224. doi: 10.1093/jme/tjae121. PMID: 38562865 Free PMC article. Updated. Preprint.
References
-
- WHO. Mosquito-borne diseases. Geneva: World Health Organization; 2020. http://www.who.int/neglected_diseases/vector_ecology/mosquito-borne-dise.... Accessed 13 Apr 2020.
-
- Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg Med Pract. 2014;16:1–23. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

