Fabrication and Microstructure of ZnO/HA Composite with In Situ Formation of Second-Phase ZnO
- PMID: 32906641
- PMCID: PMC7558110
- DOI: 10.3390/ma13183948
Fabrication and Microstructure of ZnO/HA Composite with In Situ Formation of Second-Phase ZnO
Abstract
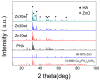
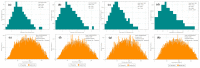
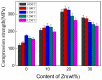
Nanometer hydroxyapatite (n-HA) powders were synthesized by the chemical precipitation method, and a novel ZnO/HA composite, which consisted of second-phase particles with different sizes and distributions, was successfully fabricated. ZnO/HA composites were prepared by using powder sintering with different Zn contents and a prefabrication pressure of 150 MPa. Microstructure and local chemical composition were analyzed by a scanning electron microscope (SEM) and energy-dispersive spectrometer (EDS), respectively. The phase composition and distribution of the composite were determined with electron back-scattered diffraction (EBSD) and an X-ray diffractometer (XRD), respectively. The experimental results of the XRD showed that the chemical precipitation method was a simple and efficient method to obtain high-purity n-HA powders. When the sintering temperature was lower than 1250 °C, the thermal stability of HA was not affected by the Zn in the sintering process. Due to sintering in an air atmosphere, the oxidation reaction of Zn took place in three stages, and ZnO as the second phase had two different sizes and distributions in the composites. The compressive strength of ZnO/HA composites, of which the highest was up to 332 MPa when the Zn content was 20%, was significantly improved compared with pure HA. The improvement in mechanical properties was mainly due to the distribution of fine ZnO particles among HA grains, which hindered the HA grain boundary migration and refinement of HA grains. As grain refinement increased the area of the grain boundary inside the material, both the grain boundary and second phase hindered crack development in different ways.
Keywords: ZnO/HA composite; compression strength; grain growth; powder sintering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Microstructures and mechanical properties of in situ TiC-β-Ti-Nb composites with ultrafine grains fabricated by high-pressure sintering.Sci Rep. 2018 Jun 22;8(1):9496. doi: 10.1038/s41598-018-27535-6. Sci Rep. 2018. PMID: 29934506 Free PMC article.
-
A Comparative Study of Pressureless Sintered Nanostructured Hydroxyapatite/TiO₂ Composites Prepared by Different TiO₂ Addition Methods.J Nanosci Nanotechnol. 2020 Apr 1;20(4):2442-2451. doi: 10.1166/jnn.2020.17213. J Nanosci Nanotechnol. 2020. PMID: 31492260
-
Improved dispersion of SiC whisker in nano hydroxyapatite and effect of atmospheres on sintering of the SiC whisker reinforced nano hydroxyapatite composites.Mater Sci Eng C Mater Biol Appl. 2018 Oct 1;91:135-145. doi: 10.1016/j.msec.2018.05.003. Epub 2018 May 3. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30033240
-
Microstructure and Mechanical Properties of Graphene-Reinforced Titanium Matrix/Nano-Hydroxyapatite Nanocomposites.Materials (Basel). 2018 Apr 16;11(4):608. doi: 10.3390/ma11040608. Materials (Basel). 2018. PMID: 29659504 Free PMC article.
-
Effects of Dopants and Processing Parameters on the Properties of ZnO-V2O5-Based Varistors Prepared by Powder Metallurgy: A Review.Materials (Basel). 2023 May 14;16(10):3725. doi: 10.3390/ma16103725. Materials (Basel). 2023. PMID: 37241352 Free PMC article. Review.
Cited by
-
Microstructure and Corrosion Behavior of Zinc/Hydroxyapatite Multi-Layer Coating Prepared by Combining Cold Spraying and High-Velocity Suspension Flame Spraying.Materials (Basel). 2023 Oct 20;16(20):6782. doi: 10.3390/ma16206782. Materials (Basel). 2023. PMID: 37895763 Free PMC article.
-
Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications.ChemMedChem. 2022 Aug 17;17(16):e202200142. doi: 10.1002/cmdc.202200142. Epub 2022 Jul 8. ChemMedChem. 2022. PMID: 35729066 Free PMC article. Review.
References
-
- Pramanik S., Agarwal A.K., Rai K., Garg A. Development of high strength hydroxyapatite by solid-state-sintering process. Ceram. Int. 2007;33:419–426. doi: 10.1016/j.ceramint.2005.10.025. - DOI
-
- Tseng Y.H., Kuo C.S., Li Y.Y., Huang C.P. Polymer-assisted synthesis of hydroxyapatite nanoparticle. Mater. Sci. Eng. C. 2009;29:819–822. doi: 10.1016/j.msec.2008.07.028. - DOI
-
- Catros S., Guillemot F., Lebraud E., Chanseau C., Perez S., Bareille R., Amédée J., Fricain J.C. Physico-chemical and biological properties of an nano-hydroxyapatite powder synthesized at room temperature. Innov. Res. Biomed. Eng. 2010;31:226–233. doi: 10.1016/j.irbm.2010.04.002. - DOI
-
- Zhang G., Chen J., Yang S., Yu Q. Preparation of amino-acid-regulated hydroxyapatite particles by hydrothermal method. Mater Lett. 2011;65:572–574. doi: 10.1016/j.matlet.2010.10.078. - DOI
-
- Ma H.B., Su W.X., Tai Z.X., Sun D.F. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin. Sci. Bull. 2012;57:3051–3058. doi: 10.1007/s11434-012-5336-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

