Use of Cell and Genome Modification Technologies to Generate Improved "Off-the-Shelf" CAR T and CAR NK Cells
- PMID: 32903482
- PMCID: PMC7438733
- DOI: 10.3389/fimmu.2020.01965
Use of Cell and Genome Modification Technologies to Generate Improved "Off-the-Shelf" CAR T and CAR NK Cells
Abstract
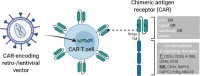
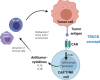
The broad success of adoptive immunotherapy to treat human cancer has resulted in a paradigm shift in modern medicine. Modification of autologous and allogenic immune cells with chimeric antigen receptors (CAR) designed to target specific antigens on tumor cells has led to production of CAR T and CAR NK cell therapies, which are ever more commonly introduced into cancer patient treatment protocols. While allogenic T cells may offer advantages such as improved anti-tumor activity, they also carry the risk of adverse reactions like graft-versus-host disease. This risk can be mitigated by use of autologous immune cells, however, the time needed for T and/or NK cell isolation, modification and expansion may be too long for some patients. Thus, there is an urgent need for strategies to robustly produce "off-the-shelf" CAR T and CAR NK cells, which could be used as a bridging therapy between cancer diagnosis or relapse and allogeneic transplantation. Advances in genome modification technologies have accelerated the generation of designer cell therapy products, including development of "off-the-shelf" CAR T cells for cancer immunotherapy. The feasibility and safety of such approaches is currently tested in clinical trials. This review will describe cell sources for CAR-based therapies, provide background of current genome editing techniques and the applicability of these approaches for generation of universal "off-the-shelf" CAR T and NK cell therapeutics.
Keywords: CRISPR-Cas9; T cell; chimeric antigen receptor; genome editing; immunotherapy.
Copyright © 2020 Morgan, Büning, Sauer and Schambach.
Figures

Similar articles
-
CAR-NK cells: A promising cellular immunotherapy for cancer.EBioMedicine. 2020 Sep;59:102975. doi: 10.1016/j.ebiom.2020.102975. Epub 2020 Aug 24. EBioMedicine. 2020. PMID: 32853984 Free PMC article. Review.
-
Engineering the next generation of CAR-NK immunotherapies.Int J Hematol. 2021 Nov;114(5):554-571. doi: 10.1007/s12185-021-03209-4. Epub 2021 Aug 28. Int J Hematol. 2021. PMID: 34453686 Free PMC article. Review.
-
Engineering strategies to safely drive CAR T-cells into the future.Front Immunol. 2024 Jun 19;15:1411393. doi: 10.3389/fimmu.2024.1411393. eCollection 2024. Front Immunol. 2024. PMID: 38962002 Free PMC article. Review.
-
Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells.Semin Immunopathol. 2019 Jan;41(1):59-68. doi: 10.1007/s00281-018-0721-x. Epub 2018 Oct 25. Semin Immunopathol. 2019. PMID: 30361801 Review.
-
Engineering Natural Killer Cells for Cancer Immunotherapy.Mol Ther. 2017 Aug 2;25(8):1769-1781. doi: 10.1016/j.ymthe.2017.06.012. Epub 2017 Jun 28. Mol Ther. 2017. PMID: 28668320 Free PMC article. Review.
Cited by
-
Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies.Front Immunol. 2022 Aug 12;13:910595. doi: 10.3389/fimmu.2022.910595. eCollection 2022. Front Immunol. 2022. PMID: 36045670 Free PMC article. Review.
-
Lateral plate mesoderm cell-based organoid system for NK cell regeneration from human pluripotent stem cells.Cell Discov. 2022 Nov 8;8(1):121. doi: 10.1038/s41421-022-00467-2. Cell Discov. 2022. PMID: 36344493 Free PMC article.
-
Modern Advances in CARs Therapy and Creating a New Approach to Future Treatment.Int J Mol Sci. 2022 Nov 30;23(23):15006. doi: 10.3390/ijms232315006. Int J Mol Sci. 2022. PMID: 36499331 Free PMC article. Review.
-
Immune Tolerance vs. Immune Resistance: The Interaction Between Host and Pathogens in Infectious Diseases.Front Vet Sci. 2022 Mar 29;9:827407. doi: 10.3389/fvets.2022.827407. eCollection 2022. Front Vet Sci. 2022. PMID: 35425833 Free PMC article.
-
Harnessing the Potential of Chimeric Antigen Receptor T-Cell Therapy for the Treatment of T-Cell Malignancies: A Dare or Double Dare?Cells. 2022 Dec 8;11(24):3971. doi: 10.3390/cells11243971. Cells. 2022. PMID: 36552738 Free PMC article. Review.
References
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. and et al.: graft-versus-leukemia reactions after bone marrow transplantation. Blood. (1990) 75:555–62. - PubMed
-
- Samelson LE, Donovan JA, Isakov N, Ota Y, Wange RL. Signal transduction mediated by the T-cell antigen receptor. Ann N Y Acad Sci. (1995) 766:157–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

