Isoquercitrin Delays Denervated Soleus Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation
- PMID: 32903465
- PMCID: PMC7435639
- DOI: 10.3389/fphys.2020.00988
Isoquercitrin Delays Denervated Soleus Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation
Abstract
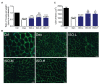
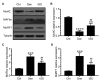
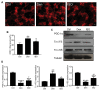
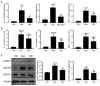
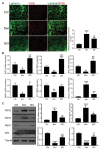
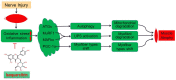
Although denervated muscle atrophy is common, the underlying molecular mechanism remains unelucidated. We have previously found that oxidative stress and inflammatory response may be early events that trigger denervated muscle atrophy. Isoquercitrin is a biologically active flavonoid with antioxidative and anti-inflammatory properties. The present study investigated the effect of isoquercitrin on denervated soleus muscle atrophy and its possible molecular mechanisms. We found that isoquercitrin was effective in alleviating soleus muscle mass loss following denervation in a dose-dependent manner. Isoquercitrin demonstrated the optimal protective effect at 20 mg/kg/d, which was the dose used in subsequent experiments. To further explore the protective effect of isoquercitrin on denervated soleus muscle atrophy, we analyzed muscle proteolysis via the ubiquitin-proteasome pathway, mitophagy, and muscle fiber type conversion. Isoquercitrin significantly inhibited the denervation-induced overexpression of two muscle-specific ubiquitin ligases-muscle RING finger 1 (MuRF1) and muscle atrophy F-box (MAFbx), and reduced the degradation of myosin heavy chains (MyHCs) in the target muscle. Following isoquercitrin treatment, mitochondrial vacuolation and autophagy were inhibited, as evidenced by reduced level of autophagy-related proteins (ATG7, BNIP3, LC3B, and PINK1); slow-to-fast fiber type conversion in the target muscle was delayed via triggering expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α); and the production of reactive oxygen species (ROS) in the target muscle was reduced, which might be associated with the upregulation of antioxidant factors (SOD1, SOD2, NRF2, NQO1, and HO1) and the downregulation of ROS production-related factors (Nox2, Nox4, and DUOX1). Furthermore, isoquercitrin treatment reduced the levels of inflammatory factors-interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α)-in the target muscle and inactivated the JAK/STAT3 signaling pathway. Overall, isoquercitrin may alleviate soleus muscle atrophy and mitophagy and reverse the slow-to-fast fiber type conversion following denervation via inhibition of oxidative stress and inflammatory response. Our study findings enrich the knowledge regarding the molecular regulatory mechanisms of denervated muscle atrophy and provide a scientific basis for isoquercitrin as a protective drug for the prevention and treatment of denervated muscle atrophy.
Keywords: denervated muscle atrophy; inflammation; isoquercitrin; mitophagy; oxidative stress; proteolysis.
Copyright © 2020 Shen, Zhang, Huang, Zhu, Qiu, Ma, Yang, Ding and Sun.
Figures

Similar articles
-
Aspirin alleviates denervation-induced muscle atrophy via regulating the Sirt1/PGC-1α axis and STAT3 signaling.Ann Transl Med. 2020 Nov;8(22):1524. doi: 10.21037/atm-20-5460. Ann Transl Med. 2020. PMID: 33313269 Free PMC article.
-
SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation.Antioxidants (Basel). 2021 Dec 28;11(1):66. doi: 10.3390/antiox11010066. Antioxidants (Basel). 2021. PMID: 35052570 Free PMC article.
-
Astragaloside IV Improves Muscle Atrophy by Modulating the Activity of UPS and ALP via Suppressing Oxidative Stress and Inflammation in Denervated Mice.Mol Neurobiol. 2024 Oct 31. doi: 10.1007/s12035-024-04590-x. Online ahead of print. Mol Neurobiol. 2024. PMID: 39480556
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article. Review.
-
Mitochondrial dysregulation and muscle disuse atrophy.F1000Res. 2019 Sep 11;8:F1000 Faculty Rev-1621. doi: 10.12688/f1000research.19139.1. eCollection 2019. F1000Res. 2019. PMID: 31559011 Free PMC article. Review.
Cited by
-
Celecoxib ameliorates diabetic sarcopenia by inhibiting inflammation, stress response, mitochondrial dysfunction, and subsequent activation of the protein degradation systems.Front Pharmacol. 2024 Jan 19;15:1344276. doi: 10.3389/fphar.2024.1344276. eCollection 2024. Front Pharmacol. 2024. PMID: 38313305 Free PMC article.
-
Acute glucose fluctuation promotes in vitro intestinal epithelial cell apoptosis and inflammation via the NOX4/ROS/JAK/STAT3 signaling pathway.Exp Ther Med. 2021 Jul;22(1):688. doi: 10.3892/etm.2021.10120. Epub 2021 Apr 28. Exp Ther Med. 2021. PMID: 33986853 Free PMC article.
-
ROS-activated CXCR2+ neutrophils recruited by CXCL1 delay denervated skeletal muscle atrophy and undergo P53-mediated apoptosis.Exp Mol Med. 2022 Jul;54(7):1011-1023. doi: 10.1038/s12276-022-00805-0. Epub 2022 Jul 21. Exp Mol Med. 2022. PMID: 35864308 Free PMC article.
-
Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration.Int J Mol Sci. 2024 May 27;25(11):5811. doi: 10.3390/ijms25115811. Int J Mol Sci. 2024. PMID: 38892005 Free PMC article. Review.
-
Differences in susceptibility to ADR nephropathy among C57BL/6 substrains.Exp Anim. 2023 Nov 9;72(4):520-525. doi: 10.1538/expanim.23-0003. Epub 2023 Jun 21. Exp Anim. 2023. PMID: 37344407 Free PMC article.
References
-
- Arouche-Delaperche L., Allenbach Y., Amelin D., Preusse C., Mouly V., Mauhin W., et al. . (2017). Pathogenic role of anti-signal recognition protein and anti-3-hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann. Neurol. 81, 538–548. 10.1002/ana.24902, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

