Association of Genetic Variants at TRPC6 With Chemotherapy-Related Heart Failure
- PMID: 32903434
- PMCID: PMC7438395
- DOI: 10.3389/fcvm.2020.00142
Association of Genetic Variants at TRPC6 With Chemotherapy-Related Heart Failure
Abstract
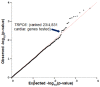
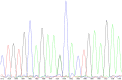
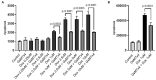
Background: Our previous GWAS identified genetic variants at six novel loci that were associated with a decline in left ventricular ejection fraction (LVEF), p < 1 × 10-5 in 1,191 early breast cancer patients from the N9831 clinical trial of chemotherapy plus trastuzumab. In this study we sought replication of these loci. Methods: We tested the top loci from the GWAS for association with chemotherapy-related heart failure (CRHF) using 26 CRHF cases from N9831 and 984 patients from the Mayo Clinic Biobank which included CRHF cases (N = 12) and control groups of patients treated with anthracycline +/- trastuzumab without HF (N = 282) and patients with HF that were never treated with anthracycline or trastuzumab (N = 690). We further examined associated loci in the context of gene expression and rare coding variants using a TWAS approach in heart left ventricle and Sanger sequencing, respectively. Doxorubicin-induced apoptosis and cardiomyopathy was modeled in human iPSC-derived cardiomyocytes and endothelial cells and a mouse model, respectively, that were pre-treated with GsMTx-4, an inhibitor of TRPC6. Results: TRPC6 5' flanking variant rs57242572-T was significantly more frequent in cases compared to controls, p = 0.031, and rs61918162-T showed a trend for association, p = 0.065. The rs61918162 T-allele was associated with higher TRPC6 expression in the heart left ventricle. We identified a single TRPC6 rare missense variant (rs767086724, N338S, prevalence 0.0025% in GnomAD) in one of 38 patients (2.6%) with CRHF. Pre-treatment of cardiomyocytes and endothelial cells with GsMTx4 significantly reduced doxorubicin-induced apoptosis. Similarly, mice treated with GsMTx4 had significantly improved doxorubicin-induced cardiac dysfunction. Conclusions: Genetic variants that are associated with increased TRPC6 expression in the heart and rare TRPC6 missense variants may be clinically useful as risk factors for CRHF. GsMTx-4 may be a cardioprotective agent in patients with TRPC6 risk variants. Replication of the genetic associations in larger well-characterized samples and functional studies are required.
Keywords: GsMTx-4; TWAS; anthracycline; breast cancer; cardiomyopathy; cardiotoxicity; doxorubicin; trastuzumab.
Copyright © 2020 Norton, Crook, Wang, Olson, Kachergus, Serie, Necela, Borgman, Advani, Ray, Landolfo, Di Florio, Hill, Bruno and Fairweather.
Figures
Similar articles
-
Replication of genetic associations of chemotherapy-related cardiotoxicity in the adjuvant NSABP B-31 clinical trial.Front Oncol. 2023 May 25;13:1139347. doi: 10.3389/fonc.2023.1139347. eCollection 2023. Front Oncol. 2023. PMID: 37305569 Free PMC article.
-
Inter-Individual Variation and Cardioprotection in Anthracycline-Induced Heart Failure.J Clin Med. 2021 Sep 9;10(18):4079. doi: 10.3390/jcm10184079. J Clin Med. 2021. PMID: 34575190 Free PMC article. Review.
-
TRPC6 N338S is a gain-of-function mutant identified in patient with doxorubicin-induced cardiotoxicity.Biochim Biophys Acta Mol Basis Dis. 2022 Nov 1;1868(11):166505. doi: 10.1016/j.bbadis.2022.166505. Epub 2022 Jul 23. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 35882306 Free PMC article.
-
Genome-wide association study of cardiotoxicity in the NCCTG N9831 (Alliance) adjuvant trastuzumab trial.Pharmacogenet Genomics. 2017 Oct;27(10):378-385. doi: 10.1097/FPC.0000000000000302. Pharmacogenet Genomics. 2017. PMID: 28763429 Free PMC article.
-
Effect of prophylactic betablocker or ACE inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy ± trastuzumab.Breast. 2018 Feb;37:64-71. doi: 10.1016/j.breast.2017.10.010. Epub 2017 Nov 1. Breast. 2018. PMID: 29101824 Review.
Cited by
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
-
Replication of genetic associations of chemotherapy-related cardiotoxicity in the adjuvant NSABP B-31 clinical trial.Front Oncol. 2023 May 25;13:1139347. doi: 10.3389/fonc.2023.1139347. eCollection 2023. Front Oncol. 2023. PMID: 37305569 Free PMC article.
-
Translational Genomic Research: The Association between Genetic Profiles and Cognitive Functioning or Cardiac Function Among Breast Cancer Survivors Completing Chemotherapy.Biol Res Nurs. 2022 Oct;24(4):433-447. doi: 10.1177/10998004221094386. Epub 2022 May 2. Biol Res Nurs. 2022. PMID: 35499926 Free PMC article.
-
Inter-Individual Variation and Cardioprotection in Anthracycline-Induced Heart Failure.J Clin Med. 2021 Sep 9;10(18):4079. doi: 10.3390/jcm10184079. J Clin Med. 2021. PMID: 34575190 Free PMC article. Review.
-
Trpc6 Promotes Doxorubicin-Induced Cardiomyopathy in Male Mice With Pleiotropic Differences Between Males and Females.Front Cardiovasc Med. 2022 Jan 13;8:757784. doi: 10.3389/fcvm.2021.757784. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35096991 Free PMC article.
References
-
- Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Jr, et al. . Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2012) 30:3792–9. 10.1200/JCO.2011.40.0010 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

