Sodium Fluctuations in Astroglia and Their Potential Impact on Astrocyte Function
- PMID: 32903427
- PMCID: PMC7435049
- DOI: 10.3389/fphys.2020.00871
Sodium Fluctuations in Astroglia and Their Potential Impact on Astrocyte Function
Abstract
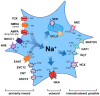
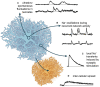
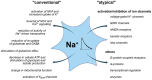
Astrocytes are the main cell type responsible for the regulation of brain homeostasis, including the maintenance of ion gradients and neurotransmitter clearance. These processes are tightly coupled to changes in the intracellular sodium (Na+) concentration. While activation of the sodium-potassium-ATPase (NKA) in response to an elevation of extracellular K+ may decrease intracellular Na+, the cotransport of transmitters, such as glutamate, together with Na+ results in an increase in astrocytic Na+. This increase in intracellular Na+ can modulate, for instance, metabolic downstream pathways. Thereby, astrocytes are capable to react on a fast time scale to surrounding neuronal activity via intracellular Na+ fluctuations and adjust energy production to the demand of their environment. Beside the well-documented conventional roles of Na+ signaling mainly mediated through changes in its electrochemical gradient, several recent studies have identified more atypical roles for Na+, including protein interactions leading to changes in their biochemical activity or Na+-dependent regulation of gene expression. In this review, we will address both the conventional as well as the atypical functions of astrocytic Na+ signaling, presenting the role of transporters and channels involved and their implications for physiological processes in the central nervous system (CNS). We will also discuss how these important functions are affected under pathological conditions, including stroke and migraine. We postulate that Na+ is an essential player not only in the maintenance of homeostatic processes but also as a messenger for the fast communication between neurons and astrocytes, adjusting the functional properties of various cellular interaction partners to the needs of the surrounding network.
Keywords: astrocyte; brain; gamma-aminobutyric acid; glutamate; ischemia; sodium imaging; sodium signaling; sodium/potassium-ATPase.
Copyright © 2020 Felix, Delekate, Petzold and Rose.
Figures

Similar articles
-
Principles of sodium homeostasis and sodium signalling in astroglia.Glia. 2016 Oct;64(10):1611-27. doi: 10.1002/glia.22964. Epub 2016 Feb 26. Glia. 2016. PMID: 26919326 Review.
-
Glutamate transporter activity promotes enhanced Na+ /K+ -ATPase-mediated extracellular K+ management during neuronal activity.J Physiol. 2016 Nov 15;594(22):6627-6641. doi: 10.1113/JP272531. Epub 2016 Jun 29. J Physiol. 2016. PMID: 27231201 Free PMC article.
-
Application of Novel Therapeutic Agents for CNS Injury: NAAG Peptidase Inhibitors.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 38. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 38. PMID: 26269888 Free Books & Documents. Review.
-
Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions.Glia. 2013 Aug;61(8):1191-205. doi: 10.1002/glia.22492. Epub 2013 Apr 2. Glia. 2013. PMID: 23553639 Review.
-
Computational Flux Balance Analysis Predicts that Stimulation of Energy Metabolism in Astrocytes and their Metabolic Interactions with Neurons Depend on Uptake of K+ Rather than Glutamate.Neurochem Res. 2017 Jan;42(1):202-216. doi: 10.1007/s11064-016-2048-0. Epub 2016 Sep 14. Neurochem Res. 2017. PMID: 27628293 Free PMC article.
Cited by
-
Dysregulation of Ion Channels and Transporters and Blood-Brain Barrier Dysfunction in Alzheimer's Disease and Vascular Dementia.Aging Dis. 2024 Aug 1;15(4):1748-1770. doi: 10.14336/AD.2023.1201. Aging Dis. 2024. PMID: 38300642 Free PMC article. Review.
-
The short form of the SUR1 and its functional implications in the damaged brain.Neural Regen Res. 2022 Mar;17(3):488-496. doi: 10.4103/1673-5374.320967. Neural Regen Res. 2022. PMID: 34380876 Free PMC article. Review.
-
Higher sodium in older individuals or after stroke/reperfusion, but not in migraine or Alzheimer's disease - a study in different preclinical models.Sci Rep. 2024 Sep 16;14(1):21636. doi: 10.1038/s41598-024-72280-8. Sci Rep. 2024. PMID: 39284837 Free PMC article.
-
Fluid transport in the brain.Physiol Rev. 2022 Apr 1;102(2):1025-1151. doi: 10.1152/physrev.00031.2020. Epub 2021 May 5. Physiol Rev. 2022. PMID: 33949874 Free PMC article. Review.
-
Approaches to Study Gap Junctional Coupling.Front Cell Neurosci. 2021 Mar 10;15:640406. doi: 10.3389/fncel.2021.640406. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33776652 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

