Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells
- PMID: 32902139
- PMCID: PMC8246936
- DOI: 10.1111/febs.15555
Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells
Abstract
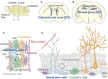
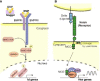
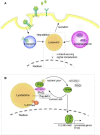
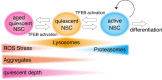
Quiescence is a cellular strategy for maintaining somatic stem cells in a specific niche in a low metabolic state without senescence for a long period of time. During development, neural stem cells (NSCs) actively proliferate and self-renew, and their progeny differentiate into both neurons and glial cells to form mature brain tissues. On the other hand, most NSCs in the adult brain are quiescent and arrested in G0/G1 phase of the cell cycle. Quiescence is essential in order to avoid the precocious exhaustion of NSCs, ensuring a sustainable source of available stem cells in the brain throughout the lifespan. After receiving activation signals, quiescent NSCs reenter the cell cycle and generate new neurons. This switching between quiescence and proliferation is tightly regulated by diverse signaling pathways. Recent studies suggest significant involvement of cellular proteostasis (homeostasis of the proteome) in the quiescent state of NSCs. Proteostasis is the result of integrated regulation of protein synthesis, folding, and degradation. In this review, we discuss regulation of quiescence by multiple signaling pathways, especially bone morphogenetic protein and Notch signaling, and focus on the functional involvement of the lysosome, an organelle governing cellular degradation, in quiescence of adult NSCs.
Keywords: adult neural stem cell; lysosome; proteostasis; quiescence; signaling.
© 2020 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Protein S Regulates Neural Stem Cell Quiescence and Neurogenesis.Stem Cells. 2017 Mar;35(3):679-693. doi: 10.1002/stem.2522. Epub 2016 Nov 8. Stem Cells. 2017. PMID: 27753164
-
Distinct Molecular Signatures of Quiescent and Activated Adult Neural Stem Cells Reveal Specific Interactions with Their Microenvironment.Stem Cell Reports. 2018 Aug 14;11(2):565-577. doi: 10.1016/j.stemcr.2018.06.005. Epub 2018 Jul 5. Stem Cell Reports. 2018. PMID: 29983386 Free PMC article.
-
LRIG1 is a gatekeeper to exit from quiescence in adult neural stem cells.Nat Commun. 2021 May 10;12(1):2594. doi: 10.1038/s41467-021-22813-w. Nat Commun. 2021. PMID: 33972529 Free PMC article.
-
Protein homeostasis and degradation in quiescent neural stem cells.J Biochem. 2024 Apr 29;175(5):481-486. doi: 10.1093/jb/mvae006. J Biochem. 2024. PMID: 38299708 Review.
-
FoxOs in neural stem cell fate decision.Arch Biochem Biophys. 2013 Jun;534(1-2):55-63. doi: 10.1016/j.abb.2012.07.017. Epub 2012 Aug 10. Arch Biochem Biophys. 2013. PMID: 22902436 Review.
Cited by
-
Epigenetic regulation in adult neural stem cells.Front Cell Dev Biol. 2024 Jan 31;12:1331074. doi: 10.3389/fcell.2024.1331074. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38357000 Free PMC article. Review.
-
Mitochondria and Other Organelles in Neural Development and Their Potential as Therapeutic Targets in Neurodegenerative Diseases.Front Neurosci. 2022 Apr 5;16:853911. doi: 10.3389/fnins.2022.853911. eCollection 2022. Front Neurosci. 2022. PMID: 35450015 Free PMC article. Review.
-
Lysosomes in Stem Cell Quiescence: A Potential Therapeutic Target in Acute Myeloid Leukemia.Cancers (Basel). 2022 Mar 23;14(7):1618. doi: 10.3390/cancers14071618. Cancers (Basel). 2022. Retraction in: Cancers (Basel). 2024 Jun 25;16(13):2317. doi: 10.3390/cancers16132317 PMID: 35406389 Free PMC article. Retracted. Review.
-
The Networking Brain: How Extracellular Matrix, Cellular Networks, and Vasculature Shape the In Vivo Mechanical Properties of the Brain.Adv Sci (Weinh). 2024 Aug;11(31):e2402338. doi: 10.1002/advs.202402338. Epub 2024 Jun 14. Adv Sci (Weinh). 2024. PMID: 38874205 Free PMC article. Review.
-
Epigenetic Regulation of Neural Stem Cells in Developmental and Adult Stages.Epigenomes. 2024 Jun 4;8(2):22. doi: 10.3390/epigenomes8020022. Epigenomes. 2024. PMID: 38920623 Free PMC article. Review.
References
-
- Altman J & Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neur 124, 319–336. - PubMed
-
- Doetsch F, Caille I, Lim DA, Garcia‐Verdugo JM & Alvarez‐Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716. - PubMed
-
- Eriksson PS, Perfilieva E, Björk‐Eriksson T, Alborn M, Nordborg C, Peterson DA & Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4, 1313–1317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

