Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules
- PMID: 32899889
- PMCID: PMC7555323
- DOI: 10.3390/antiox9090830
Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules
Abstract
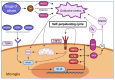
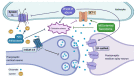
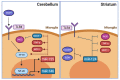
Drug abuse is a major global health and economic problem. However, there are no pharmacological treatments to effectively reduce the compulsive use of most drugs of abuse. Despite exerting different mechanisms of action, all drugs of abuse promote the activation of the brain reward system, with lasting neurobiological consequences that potentiate subsequent consumption. Recent evidence shows that the brain displays marked oxidative stress and neuroinflammation following chronic drug consumption. Brain oxidative stress and neuroinflammation disrupt glutamate homeostasis by impairing synaptic and extra-synaptic glutamate transport, reducing GLT-1, and system Xc- activities respectively, which increases glutamatergic neurotransmission. This effect consolidates the relapse-promoting effect of drug-related cues, thus sustaining drug craving and subsequent drug consumption. Recently, promising results as experimental treatments to reduce drug consumption and relapse have been shown by (i) antioxidant and anti-inflammatory synthetic molecules whose effects reach the brain; (ii) natural biomolecules secreted by mesenchymal stem cells that excel in antioxidant and anti-inflammatory properties, delivered via non-invasive intranasal administration to animal models of drug abuse and (iii) potent anti-inflammatory microRNAs and anti-miRNAs which target the microglia and reduce neuroinflammation and drug craving. In this review, we address the neurobiological consequences of brain oxidative stress and neuroinflammation that follow the chronic consumption of most drugs of abuse, and the current and potential therapeutic effects of antioxidants and anti-inflammatory agents and biomolecules to reduce these drug-induced alterations and to prevent relapse.
Keywords: drug addiction; neuroinflammation; oxidative stress; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking.Addict Biol. 2019 Sep;24(5):994-1007. doi: 10.1111/adb.12675. Epub 2018 Sep 21. Addict Biol. 2019. PMID: 30239077
-
Intranasal mesenchymal stem cell secretome administration markedly inhibits alcohol and nicotine self-administration and blocks relapse-intake: mechanism and translational options.Stem Cell Res Ther. 2019 Jul 8;10(1):205. doi: 10.1186/s13287-019-1304-z. Stem Cell Res Ther. 2019. PMID: 31286996 Free PMC article.
-
Mesenchymal stem cells as a promising therapy for alcohol use disorder.Int Rev Neurobiol. 2024;178:179-211. doi: 10.1016/bs.irn.2024.07.002. Epub 2024 Jul 25. Int Rev Neurobiol. 2024. PMID: 39523054 Review.
-
Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction.Front Cell Neurosci. 2015 Jan 20;8:466. doi: 10.3389/fncel.2014.00466. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25653591 Free PMC article. Review.
-
Main path and byways: non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission.J Neurochem. 2015 Dec;135(6):1062-79. doi: 10.1111/jnc.13348. Epub 2015 Sep 29. J Neurochem. 2015. PMID: 26336934 Free PMC article. Review.
Cited by
-
Gut Microbiome-Mediated Mechanisms in Alleviating Opioid Addiction with Aqueous Extract of Anacyclus pyrethrum.Biomedicines. 2024 May 23;12(6):1152. doi: 10.3390/biomedicines12061152. Biomedicines. 2024. PMID: 38927359 Free PMC article.
-
Deaddicta® for maintenance treatment of Opioid-dependence: A six-month follow-up.Caspian J Intern Med. 2024 Spring;15(2):318-327. doi: 10.22088/cjim.15.2.318. Caspian J Intern Med. 2024. PMID: 38807734 Free PMC article.
-
Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters' Tissue Content in Rats' Hippocampus and Amygdala.Metabolites. 2023 Apr 4;13(4):515. doi: 10.3390/metabo13040515. Metabolites. 2023. PMID: 37110173 Free PMC article.
-
Psychostimulants influence oxidative stress and redox signatures: the role of DNA methylation.Redox Rep. 2022 Dec;27(1):53-59. doi: 10.1080/13510002.2022.2043224. Redox Rep. 2022. PMID: 35227168 Free PMC article. Review.
-
Clinical Variables and Peripheral Biomarkers Associated with Substance-Induced Psychotic Disorder: Differences Related to Alcohol, Cannabis, and Psychostimulant Abuse.J Pers Med. 2024 Mar 21;14(3):325. doi: 10.3390/jpm14030325. J Pers Med. 2024. PMID: 38541067 Free PMC article.
References
-
- WHO . Global Status Report on Alcohol and Health 2018. World Health Organization; Geneva, Switzerland: 2019.
-
- WHO . WHO Report on the Global Tobacco Epidemic 2019: Offer Help to Quit Tobacco Use. World Health Organization; Geneva, Switzerland: 2019.
-
- UNODC . World Drug Report 2019. UNODC; Vienna, Austria: 2019.
-
- Van Skike C., Maggio S., Reynolds A., Casey E., Bardo M., Dwoskin L., Prendergast M., Nixon K. Critical needs in drug discovery for cessation of alcohol and nicotine polysubstance abuse. Progress Neuro Psychopharmacol. Biol. Psychiatr. 2016;65:269–287. doi: 10.1016/j.pnpbp.2015.11.004. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

