Neuroprotection by Neurotropin through Crosstalk of Neurotrophic and Innate Immune Receptors in PC12 Cells
- PMID: 32899630
- PMCID: PMC7555716
- DOI: 10.3390/ijms21186456
Neuroprotection by Neurotropin through Crosstalk of Neurotrophic and Innate Immune Receptors in PC12 Cells
Abstract
Infected or damaged tissues release multiple "alert" molecules such as alarmins and damage-associated molecular patterns (DAMPs) that are recognized by innate immune receptors, and induce tissue inflammation, regeneration, and repair. Recently, an extract from inflamed rabbit skin inoculated with vaccinia virus (Neurotropin®, NTP) was found to induce infarct tolerance in mice receiving permanent ischemic attack to the middle cerebral artery. Likewise, we report herein that NTP prevented the neurite retraction in PC12 cells by nerve growth factor (NGF) deprivation. This effect was accompanied by interaction of Fyn with high-affinity NGF receptor TrkA. Sucrose density gradient subcellular fractionation of NTP-treated cells showed heretofore unidentified membrane fractions with a high-buoyant density containing Trk, B subunit of cholera toxin-bound ganglioside, flotillin-1 and Fyn. Additionally, these new membrane fractions also contained Toll-like receptor 4 (TLR4). Inhibition of TLR4 function by TAK-242 prevented the formation of these unidentified membrane fractions and suppressed neuroprotection by NTP. These observations indicate that NTP controls TrkA-mediated signaling through the formation of clusters of new membrane microdomains, thus providing a platform for crosstalk between neurotrophic and innate immune receptors. Neuroprotective mechanisms through the interaction with innate immune systems may provide novel mechanism for neuroprotection.
Keywords: Fyn; GM1 ganglioside (GM1); Neurotropin; Toll-like receptor4 (TLR4); TrkA tyrosine kinase; lipid rafts.
Conflict of interest statement
The affiliations of each author are noted in the citation appended to the list of authors. The authors disclose the following industry relationships: Y.F. is a full-time employee of Nippon Zoki Pharmaceutical Co., Ltd. All the experimental works included in this article was performed by Y.F., K.N., T.M. All authors reported no COI to the present study.
Figures
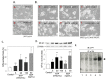


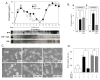
Similar articles
-
Neurotropin promotes NGF signaling through interaction of GM1 ganglioside with Trk neurotrophin receptor in PC12 cells.Brain Res. 2015 Jan 30;1596:13-21. doi: 10.1016/j.brainres.2014.11.041. Epub 2014 Nov 29. Brain Res. 2015. PMID: 25454796
-
Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells.J Biol Chem. 2004 Aug 6;279(32):33368-78. doi: 10.1074/jbc.M403816200. Epub 2004 May 15. J Biol Chem. 2004. PMID: 15145933
-
Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein.Mol Cell Biol. 2007 Aug;27(16):5686-98. doi: 10.1128/MCB.01109-06. Epub 2007 Jun 4. Mol Cell Biol. 2007. PMID: 17548467 Free PMC article.
-
NGF and Its Receptors in the Regulation of Inflammatory Response.Int J Mol Sci. 2017 May 11;18(5):1028. doi: 10.3390/ijms18051028. Int J Mol Sci. 2017. PMID: 28492466 Free PMC article. Review.
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
Cited by
-
Post-Event Application of Neurotropin Protects against Ischemic Insult toward Better Outcomes in a Murine Model of Subarachnoid Hemorrhage.Biomedicines. 2021 Jun 10;9(6):664. doi: 10.3390/biomedicines9060664. Biomedicines. 2021. PMID: 34200698 Free PMC article.
-
Editorial for Special Issue "Gangliosides: Modes of Action and Cell Fates".Int J Mol Sci. 2020 Sep 8;21(18):6552. doi: 10.3390/ijms21186552. Int J Mol Sci. 2020. PMID: 32911611 Free PMC article.
-
Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling.Int J Mol Sci. 2021 Sep 3;22(17):9565. doi: 10.3390/ijms22179565. Int J Mol Sci. 2021. PMID: 34502474 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

