Umbilical Cord Mesenchymal Stem Cell-Derived Nanovesicles Potentiate the Bone-Formation Efficacy of Bone Morphogenetic Protein 2
- PMID: 32899307
- PMCID: PMC7504262
- DOI: 10.3390/ijms21176425
Umbilical Cord Mesenchymal Stem Cell-Derived Nanovesicles Potentiate the Bone-Formation Efficacy of Bone Morphogenetic Protein 2
Abstract
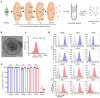
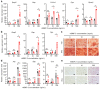
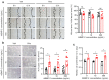
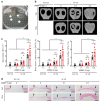
Recombinant human bone morphogenetic protein 2 (rhBMP-2) is one of the most potent osteogenic factors used to treat bone loss. However, at higher doses, rhBMP-2 does not necessarily increase bone formation but rather increases the incidence of adverse side effects. Here, we investigated whether umbilical cord mesenchymal stem cell (UCMSC)-derived nanovesicles (NVs) further increase the in vivo bone formation at high doses of rhBMP-2. In the presence of UCMSC-derived NVs, proliferation, migration, and tube formation of human umbilical vein endothelial cells were stimulated in vitro. Furthermore, migration and osteogenesis of human bone marrow-derived mesenchymal stem cells were stimulated. To examine the efficacy of UCMSC-derived NVs on in vivo bone formation, collagen sponges soaked with rhBMP-2 and UCMSC-derived NVs were used in athymic nude mice with calvarial defects. At a high rhBMP-2 dosage (500 ng/mL), UCMSC-derived NVs significantly promoted bone formation in calvarial defects; however, the UCMSC-derived NVs alone did not induce in vivo bone formation. Our results indicate that UCMSC-derived NVs can potentiate the bone formation efficacy of rhBMP-2 at a high dosage.
Keywords: angiogenesis; bone formation; osteogenesis; recombinant human bone morphogenetic protein 2; umbilical cord mesenchymal stem cell-derived nanovesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Combined Use of Recombinant Human BMP-7 and Osteogenic Media May Have No Ideal Synergistic Effect on Leporine Bone Regeneration of Human Umbilical Cord Mesenchymal Stem Cells Seeded on Nanohydroxyapatite/Collagen/Poly (l-Lactide).Stem Cells Dev. 2020 Sep 15;29(18):1215-1228. doi: 10.1089/scd.2020.0066. Epub 2020 Aug 13. Stem Cells Dev. 2020. PMID: 32674666
-
Ectopic vascularized bone formation by human umbilical cord-derived mesenchymal stromal cells expressing bone morphogenetic factor-2 and endothelial cells.Biochem Biophys Res Commun. 2018 Sep 26;504(1):302-308. doi: 10.1016/j.bbrc.2018.08.179. Epub 2018 Sep 4. Biochem Biophys Res Commun. 2018. PMID: 30190122
-
Umbilical cord tissue-derived mesenchymal stem cells grow best under GMP-compliant culture conditions and maintain their phenotypic and functional properties.J Immunol Methods. 2010 Dec 15;363(1):80-9. doi: 10.1016/j.jim.2010.10.008. Epub 2010 Oct 28. J Immunol Methods. 2010. PMID: 21035451
-
Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: From mechanism to therapeutics.Cell Prolif. 2024 Apr;57(4):e13586. doi: 10.1111/cpr.13586. Epub 2023 Dec 26. Cell Prolif. 2024. PMID: 38148579 Free PMC article. Review.
-
Mesenchymal stem cells from umbilical cord tissue as potential therapeutics for cardiomyodegenerative diseases - a review.Int J Mol Cell Med. 2012 Summer;1(3):119-32. Int J Mol Cell Med. 2012. PMID: 24551768 Free PMC article. Review.
Cited by
-
The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine.Cells. 2023 Nov 3;12(21):2567. doi: 10.3390/cells12212567. Cells. 2023. PMID: 37947645 Free PMC article.
-
Epigenetic modification: A novel insight into diabetic wound healing.Heliyon. 2024 Mar 13;10(6):e28086. doi: 10.1016/j.heliyon.2024.e28086. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38533007 Free PMC article. Review.
-
Evaluation of the regenerative capacity of stem cells combined with bone graft material and collagen matrix using a rabbit calvarial defect model.J Periodontal Implant Sci. 2023 Dec;53(6):467-477. doi: 10.5051/jpis.2204880244. Epub 2023 Mar 28. J Periodontal Implant Sci. 2023. PMID: 37154108 Free PMC article.
-
Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering.Polymers (Basel). 2021 Feb 17;13(4):599. doi: 10.3390/polym13040599. Polymers (Basel). 2021. PMID: 33671329 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

