Teaching Old Dogs New Tricks? The Plasticity of Lung Alveolar Macrophage Subsets
- PMID: 32896485
- PMCID: PMC7472979
- DOI: 10.1016/j.it.2020.08.008
Teaching Old Dogs New Tricks? The Plasticity of Lung Alveolar Macrophage Subsets
Abstract
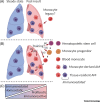
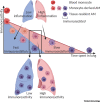
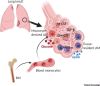
Alveolar macrophages (AMs) are highly abundant lung cells with important roles in homeostasis and immunity. Their function influences the outcome of lung infections, lung cancer, and chronic inflammatory disease. Recent findings reveal functional heterogeneity of AMs. Following lung insult, resident AMs can either remain unchanged, acquire new functionality, or be replaced by monocyte-derived AMs. Evidence from mouse models correlates AM function with their embryonic or monocyte origin. We hypothesize that resident AMs are terminally differentiated cells with low responsiveness and limited plasticity, while recruited, monocyte-derived AMs are initially highly immunoreactive but more plastic, able to change their function in response to environmental cues. Understanding cell-intrinsic and -extrinsic mechanisms determining AM function may provide opportunities for intervention in lung disease.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans.J Immunol. 2014 Mar 15;192(6):2821-9. doi: 10.4049/jimmunol.1302269. Epub 2014 Feb 17. J Immunol. 2014. PMID: 24534529 Free PMC article.
-
A Functional Assessment of Fetal Liver and Monocyte-Derived Macrophages in the Lung Alveolar Environment.J Immunol. 2024 Mar 15;212(6):1012-1021. doi: 10.4049/jimmunol.2300626. J Immunol. 2024. PMID: 38251913
-
Beryllium increases the CD14(dim)CD16+ subset in the lung of chronic beryllium disease.PLoS One. 2015 Feb 17;10(2):e0117276. doi: 10.1371/journal.pone.0117276. eCollection 2015. PLoS One. 2015. PMID: 25689051 Free PMC article.
-
Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis.Front Immunol. 2020 Oct 15;11:583042. doi: 10.3389/fimmu.2020.583042. eCollection 2020. Front Immunol. 2020. PMID: 33178214 Free PMC article. Review.
-
Does tissue imprinting restrict macrophage plasticity?Nat Immunol. 2021 Feb;22(2):118-127. doi: 10.1038/s41590-020-00849-2. Epub 2021 Jan 18. Nat Immunol. 2021. PMID: 33462453 Review.
Cited by
-
The role of lung macrophages in acute respiratory distress syndrome.Inflamm Res. 2022 Dec;71(12):1417-1432. doi: 10.1007/s00011-022-01645-4. Epub 2022 Oct 20. Inflamm Res. 2022. PMID: 36264361 Free PMC article. Review.
-
How and to What Extent Immunological Responses to SARS-CoV-2 Shape Pulmonary Function in COVID-19 Patients.Front Physiol. 2021 Jun 29;12:628288. doi: 10.3389/fphys.2021.628288. eCollection 2021. Front Physiol. 2021. PMID: 34267671 Free PMC article.
-
Gene Therapy for Occupational Lung Disease: Steering Macrophages in the Right Direction?Am J Respir Cell Mol Biol. 2023 Feb;68(2):129-130. doi: 10.1165/rcmb.2022-0408ED. Am J Respir Cell Mol Biol. 2023. PMID: 36315436 Free PMC article. No abstract available.
-
Optimized method for higher yield of alveolar macrophage isolation for ex vivo studies.Heliyon. 2024 Aug 30;10(17):e37221. doi: 10.1016/j.heliyon.2024.e37221. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39319125 Free PMC article.
-
Leukocyte Function in COPD: Clinical Relevance and Potential for Drug Therapy.Int J Chron Obstruct Pulmon Dis. 2021 Jul 30;16:2227-2242. doi: 10.2147/COPD.S266394. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34354348 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

