Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: Immunodynamic biomarkers and overall survival
- PMID: 32889778
- PMCID: PMC7648021
- DOI: 10.1111/cas.14641
Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: Immunodynamic biomarkers and overall survival
Abstract
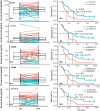
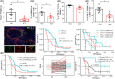
Advanced hepatitis B virus (HBV)-related hepatocellular carcinoma HCC with poor prognosis is often associated with chronic inflammation, immune tolerance, and marked heterogeneity. The interleukin-6 (IL-6)/JAK/STAT3 signal pathways play multiple regulatory roles in modulating inflammation and immunity in cancers. Polarization of myeloid-derived suppressor cells (MDSCs) is involved in HBV-related immunosuppression and CD8+ T-cell activation through ERK/IL-6/STAT3. Icaritin is a small molecule that has displayed anticancer activities through IL-6/JAK/STAT3 pathways in tumor cells and immune cells including CD8+ T cells, MDSCs, neutrophils, and macrophages. This study aimed to confirm icaritin immunomodulation in advanced HBV-related HCC patients with poor prognosis. Immunomodulation of MDSCs was evaluated in BALB/c mice in vivo. Immunomodulation of serum cytokines and a panel of immune checkpoint proteins were assessed in HBV-related, histologically confirmed HCC patients. Poor prognostic characteristics included HBV infection, bulky tumors, Child-Pugh B classification, and metastasis. Clinical end-points included safety, tumor response, and overall survival (OS). Icaritin treatment-induced dynamics of serum cytokines IL-6, IL-8, IL-10, and tumor necrosis factor-α, and soluble immune checkpoint proteins TIM3, LAG3, CD28, CD80, and CTLA-4 were assessed. No grade III/IV treatment-related adverse events were observed. Time-to-progression was significantly associated with the prognostic factors. Improved survival was observed in the advanced HCC patients with dynamic changes of cytokines, immune checkpoint proteins, and immune cells. Median OS (329-565 days) was significantly correlated with baseline hepatitis B surface antigen positivity, cytokines, tumor neoantigens, and Stenotrophomonas maltophilia infection. Composite biomarker scores of high-level α-fetoprotein and T helper type I (Th1)/Th2 cytokines associated with favorable survival warrant further clinical development of icaritin as an alternative immune-modulatory regimen to treat advanced HCC patients with poor prognosis.
Keywords: HBV-related advanced HCC; dynamic biomarker; icaritin anticancer immunomodulation; survival.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
SL is an ex‐employee and shareholder of Beijing Shenogen. BY and KM are employees and shareholders of Beijing Shenogen Biomedical Ltd. The other authors have no conflict of interest.
Figures
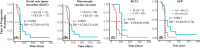

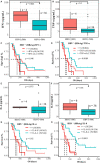
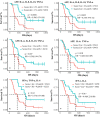
Similar articles
-
Icaritin Induces Anti-tumor Immune Responses in Hepatocellular Carcinoma by Inhibiting Splenic Myeloid-Derived Suppressor Cell Generation.Front Immunol. 2021 Feb 26;12:609295. doi: 10.3389/fimmu.2021.609295. eCollection 2021. Front Immunol. 2021. PMID: 33717093 Free PMC article.
-
First-in-class immune-modulating small molecule Icaritin in advanced hepatocellular carcinoma: preliminary results of safety, durable survival and immune biomarkers.BMC Cancer. 2019 Mar 28;19(1):279. doi: 10.1186/s12885-019-5471-1. BMC Cancer. 2019. PMID: 30922248 Free PMC article. Clinical Trial.
-
Association of TIP30 expression and prognosis of hepatocellular carcinoma in patients with HBV infection.Cancer Med. 2016 Sep;5(9):2180-9. doi: 10.1002/cam4.728. Epub 2016 Jul 15. Cancer Med. 2016. PMID: 27418384 Free PMC article.
-
New insights into the anticancer therapeutic potential of icaritin and its synthetic derivatives.Drug Dev Res. 2024 Apr;85(2):e22175. doi: 10.1002/ddr.22175. Drug Dev Res. 2024. PMID: 38567708 Review.
-
HBV-Induced Immune Imbalance in the Development of HCC.Front Immunol. 2019 Aug 27;10:2048. doi: 10.3389/fimmu.2019.02048. eCollection 2019. Front Immunol. 2019. PMID: 31507621 Free PMC article. Review.
Cited by
-
Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects.Pharmaceuticals (Basel). 2022 Mar 24;15(4):397. doi: 10.3390/ph15040397. Pharmaceuticals (Basel). 2022. PMID: 35455393 Free PMC article. Review.
-
Nucleolin recognizing silica nanoparticles inhibit cell proliferation by activating the Bax/Bcl-2/caspase-3 signalling pathway to induce apoptosis in liver cancer.Front Pharmacol. 2023 Feb 9;14:1117052. doi: 10.3389/fphar.2023.1117052. eCollection 2023. Front Pharmacol. 2023. PMID: 36843953 Free PMC article.
-
Tumor Microenvironment in Hepatocellular Carcinoma: Key Players for Immunotherapy.J Hepatocell Carcinoma. 2022 Oct 26;9:1109-1125. doi: 10.2147/JHC.S381764. eCollection 2022. J Hepatocell Carcinoma. 2022. PMID: 36320666 Free PMC article. Review.
-
Soluble immune checkpoint molecules in cancer risk, outcomes prediction, and therapeutic applications.Biomark Res. 2024 Sep 2;12(1):95. doi: 10.1186/s40364-024-00647-0. Biomark Res. 2024. PMID: 39218939 Free PMC article. Review.
-
Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets.J Exp Clin Cancer Res. 2021 May 18;40(1):172. doi: 10.1186/s13046-021-01968-w. J Exp Clin Cancer Res. 2021. PMID: 34006331 Free PMC article. Review.
References
-
- Llovet JMVA, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Cli Oncology. 2015;12:436. - PubMed
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. - PubMed
-
- Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving Sorafenib for advanced hepatocellular cancer: a meta‐analysis of randomized phase III trials. J Clin Oncol. 2017;35:622‐628. - PubMed
-
- Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18(11):827‐844. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

