Fruit setting rewires central metabolism via gibberellin cascades
- PMID: 32883877
- PMCID: PMC7519230
- DOI: 10.1073/pnas.2011859117
Fruit setting rewires central metabolism via gibberellin cascades
Abstract
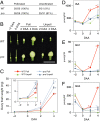
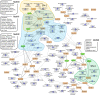
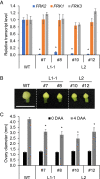
Fruit set is the process whereby ovaries develop into fruits after pollination and fertilization. The process is induced by the phytohormone gibberellin (GA) in tomatoes, as determined by the constitutive GA response mutant procera However, the role of GA on the metabolic behavior in fruit-setting ovaries remains largely unknown. This study explored the biochemical mechanisms of fruit set using a network analysis of integrated transcriptome, proteome, metabolome, and enzyme activity data. Our results revealed that fruit set involves the activation of central carbon metabolism, with increased hexoses, hexose phosphates, and downstream metabolites, including intermediates and derivatives of glycolysis, the tricarboxylic acid cycle, and associated organic and amino acids. The network analysis also identified the transcriptional hub gene SlHB15A, that coordinated metabolic activation. Furthermore, a kinetic model of sucrose metabolism predicted that the sucrose cycle had high activity levels in unpollinated ovaries, whereas it was shut down when sugars rapidly accumulated in vacuoles in fruit-setting ovaries, in a time-dependent manner via tonoplastic sugar carriers. Moreover, fruit set at least partly required the activity of fructokinase, which may pull fructose out of the vacuole, and this could feed the downstream pathways. Collectively, our results indicate that GA cascades enhance sink capacities, by up-regulating central metabolic enzyme capacities at both transcriptional and posttranscriptional levels. This leads to increased sucrose uptake and carbon fluxes for the production of the constituents of biomass and energy that are essential for rapid ovary growth during the initiation of fruit set.
Keywords: fruit set; gibberellin; metabolic enzymes; parthenocarpy; tomatoes.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Molecular, hormonal, and metabolic mechanisms of fruit set, the ovary-to-fruit transition, in horticultural crops.J Exp Bot. 2023 Oct 31;74(20):6254-6268. doi: 10.1093/jxb/erad214. J Exp Bot. 2023. PMID: 37279328 Review.
-
The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway.J Exp Bot. 2012 Oct;63(16):5803-13. doi: 10.1093/jxb/ers229. Epub 2012 Sep 3. J Exp Bot. 2012. PMID: 22945942
-
The role of ethylene in the regulation of ovary senescence and fruit set in tomato (Solanum lycopersicum).Plant Signal Behav. 2018 Apr 3;13(4):e1146844. doi: 10.1080/15592324.2016.1146844. Epub 2018 Apr 16. Plant Signal Behav. 2018. PMID: 26934126 Free PMC article.
-
Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development.Plant Physiol. 2012 Nov;160(3):1581-96. doi: 10.1104/pp.112.204552. Epub 2012 Aug 31. Plant Physiol. 2012. PMID: 22942390 Free PMC article.
-
The role of auxin and gibberellin in tomato fruit set.J Exp Bot. 2009;60(5):1523-32. doi: 10.1093/jxb/erp094. Epub 2009 Mar 25. J Exp Bot. 2009. PMID: 19321650 Review.
Cited by
-
Gibberellin in tomato: metabolism, signaling and role in drought responses.Mol Hortic. 2021 Nov 24;1(1):15. doi: 10.1186/s43897-021-00019-4. Mol Hortic. 2021. PMID: 37789477 Free PMC article. Review.
-
Mass Spectrometry Imaging of Flavonols and Ellagic Acid Glycosides in Ripe Strawberry Fruit.Molecules. 2020 Oct 9;25(20):4600. doi: 10.3390/molecules25204600. Molecules. 2020. PMID: 33050295 Free PMC article.
-
Functional Characterization of the Gibberellin (GA) Receptor ScGID1 in Sugarcane.Int J Mol Sci. 2024 Oct 4;25(19):10688. doi: 10.3390/ijms251910688. Int J Mol Sci. 2024. PMID: 39409017 Free PMC article.
-
Genetic and Molecular Mechanisms Conferring Heat Stress Tolerance in Tomato Plants.Front Plant Sci. 2021 Dec 24;12:786688. doi: 10.3389/fpls.2021.786688. eCollection 2021. Front Plant Sci. 2021. PMID: 35003175 Free PMC article. Review.
-
New Advances in the Study of Regulation of Tomato Flowering-Related Genes Using Biotechnological Approaches.Plants (Basel). 2024 Jan 25;13(3):359. doi: 10.3390/plants13030359. Plants (Basel). 2024. PMID: 38337892 Free PMC article. Review.
References
-
- Yamaguchi S., Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59, 225–251 (2008). - PubMed
-
- Ueguchi-Tanaka M., Nakajima M., Motoyuki A., Matsuoka M., Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 58, 183–198 (2007). - PubMed
-
- Bassel G. W., Mullen R. T., Bewley J. D., procera is a putative DELLA mutant in tomato (Solanum lycopersicum): Effects on the seed and vegetative plant. J. Exp. Bot. 59, 585–593 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

