Discovery of Novel pERK1/2- or β-Arrestin-Preferring 5-HT1A Receptor-Biased Agonists: Diversified Therapeutic-like versus Side Effect Profile
- PMID: 32883072
- PMCID: PMC7586344
- DOI: 10.1021/acs.jmedchem.0c00814
Discovery of Novel pERK1/2- or β-Arrestin-Preferring 5-HT1A Receptor-Biased Agonists: Diversified Therapeutic-like versus Side Effect Profile
Abstract
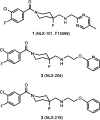
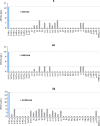
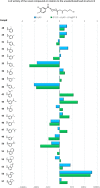
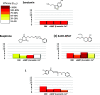
Novel 1-(1-benzoylpiperidin-4-yl)methanamine derivatives with high affinity and selectivity for serotonin 5-HT1A receptors were obtained and tested in four functional assays: ERK1/2 phosphorylation, adenylyl cyclase inhibition, calcium mobilization, and β-arrestin recruitment. Compounds 44 and 56 (2-methylaminophenoxyethyl and 2-(1H-indol-4-yloxy)ethyl derivatives, respectively) were selected as biased agonists with highly differential "signaling fingerprints" that translated into distinct in vivo profiles. In vitro, 44 showed biased agonism for ERK1/2 phosphorylation and, in vivo, it preferentially exerted an antidepressant-like effect in the Porsolt forced swimming test in rats. In contrast, compound 56 exhibited a first-in-class profile: it preferentially and potently activated β-arrestin recruitment in vitro and potently elicited lower lip retraction in vivo, a component of "serotonergic syndrome". Both compounds showed promising developability properties. The presented 5-HT1A receptor-biased agonists, preferentially targeting various signaling pathways, have the potential to become drug candidates for distinct central nervous system pathologies and possessing accentuated therapeutic activity and reduced side effects.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.N.-T. is an employee and a shareholder of Neurolixis.
Figures
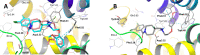
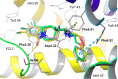



Similar articles
-
Novel Aryloxyethyl Derivatives of 1-(1-Benzoylpiperidin-4-yl)methanamine as the Extracellular Regulated Kinases 1/2 (ERK1/2) Phosphorylation-Preferring Serotonin 5-HT1A Receptor-Biased Agonists with Robust Antidepressant-like Activity.J Med Chem. 2019 Mar 14;62(5):2750-2771. doi: 10.1021/acs.jmedchem.9b00062. Epub 2019 Mar 2. J Med Chem. 2019. PMID: 30721053
-
From Receptor Selectivity to Functional Selectivity: The Rise of Biased Agonism in 5-HT1A Receptor Drug Discovery.Curr Top Med Chem. 2019;19(26):2393-2420. doi: 10.2174/1568026619666190911122040. Curr Top Med Chem. 2019. PMID: 31544717 Review.
-
Arylpiperazine agonists of the serotonin 5-HT1A receptor preferentially activate cAMP signaling versus recruitment of β-arrestin-2.Bioorg Med Chem. 2015 Aug 1;23(15):4824-4830. doi: 10.1016/j.bmc.2015.05.042. Epub 2015 Jun 1. Bioorg Med Chem. 2015. PMID: 26081758
-
Translating biased agonists from molecules to medications: Serotonin 5-HT1A receptor functional selectivity for CNS disorders.Pharmacol Ther. 2022 Jan;229:107937. doi: 10.1016/j.pharmthera.2021.107937. Epub 2021 Jun 24. Pharmacol Ther. 2022. PMID: 34174274 Review.
-
Identification of G protein-biased agonists that fail to recruit β-arrestin or promote internalization of the D1 dopamine receptor.ACS Chem Neurosci. 2015 Apr 15;6(4):681-92. doi: 10.1021/acschemneuro.5b00020. Epub 2015 Feb 20. ACS Chem Neurosci. 2015. PMID: 25660762 Free PMC article.
Cited by
-
The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference.Int J Mol Sci. 2022 Aug 8;23(15):8814. doi: 10.3390/ijms23158814. Int J Mol Sci. 2022. PMID: 35955946 Free PMC article. Review.
-
2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule.Int J Mol Sci. 2022 Apr 7;23(8):4091. doi: 10.3390/ijms23084091. Int J Mol Sci. 2022. PMID: 35456915 Free PMC article.
-
INTEDE 2.0: the metabolic roadmap of drugs.Nucleic Acids Res. 2024 Jan 5;52(D1):D1355-D1364. doi: 10.1093/nar/gkad1013. Nucleic Acids Res. 2024. PMID: 37930837 Free PMC article.
-
Novel and atypical pathways for serotonin signaling.Fac Rev. 2021 Jun 1;10:52. doi: 10.12703/r/10-52. eCollection 2021. Fac Rev. 2021. PMID: 34195691 Free PMC article. Review.
-
Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures.Molecules. 2024 Feb 22;29(5):964. doi: 10.3390/molecules29050964. Molecules. 2024. PMID: 38474476 Free PMC article. Review.
References
-
- Adell A. Lu-AA21004, a Multimodal Serotonergic Agent, for the Potential Treatment of Depression and Anxiety. IDrugs 2010, 12, 900–910. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

