Activation of GPR56, a novel adhesion GPCR, is necessary for nuclear androgen receptor signaling in prostate cells
- PMID: 32881870
- PMCID: PMC7470385
- DOI: 10.1371/journal.pone.0226056
Activation of GPR56, a novel adhesion GPCR, is necessary for nuclear androgen receptor signaling in prostate cells
Abstract
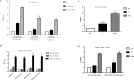
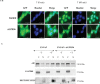
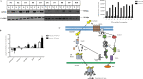
The androgen receptor (AR) is activated in patients with castration resistant prostate cancer (CRPC) despite low circulating levels of androgen, suggesting that intracellular signaling pathways and non-androgenic factors may contribute to AR activation. Many G-protein coupled receptors (GPCR) and their ligands are also activated in these cells indicating that they may play a role in development of Prostate Cancer (PCa) and CRPC. Although a cross talk has been suggested between the two pathways, yet, the identity of GPCRs which may play a role in androgen signaling, is not established yet. By using blast analysis of 826 GPCRs, we identified a GPCR, GPCR 205, which exhibited maximum similarity with the ligand binding domain of the AR. We demonstrate that adhesion GPCR 205, also known as GPR56, can be activated by androgens to stimulate the Rho signaling pathway, a pathway that plays an important role in prostate tumor cell metastasis. Testosterone stimulation of GPR56 also activates the cAMP/ Protein kinase A (PKA) pathway, that is necessary for AR signaling. Knocking down the expression of GPR56 using siRNA, disrupts nuclear translocation of AR and transcription of prototypic AR target genes such as PSA. GPR56 expression is higher in all twenty-five prostate tumor patient's samples tested and cells expressing GPR56 exhibit increased proliferation. These findings provide new insights about androgen signaling and identify GPR56 as a possible therapeutic target in advanced prostate cancer patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Regulation and targeting of androgen receptor nuclear localization in castration-resistant prostate cancer.J Clin Invest. 2021 Feb 15;131(4):e141335. doi: 10.1172/JCI141335. J Clin Invest. 2021. PMID: 33332287 Free PMC article.
-
Phosphorylation of HSP90 by protein kinase A is essential for the nuclear translocation of androgen receptor.J Biol Chem. 2019 May 31;294(22):8699-8710. doi: 10.1074/jbc.RA119.007420. Epub 2019 Apr 16. J Biol Chem. 2019. PMID: 30992362 Free PMC article.
-
The N-terminal domain of the androgen receptor drives its nuclear localization in castration-resistant prostate cancer cells.J Steroid Biochem Mol Biol. 2014 Sep;143:473-80. doi: 10.1016/j.jsbmb.2014.03.004. Epub 2014 Mar 22. J Steroid Biochem Mol Biol. 2014. PMID: 24662325 Free PMC article.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Targeting the androgen receptor signaling pathway in advanced prostate cancer.Am J Health Syst Pharm. 2022 Jul 22;79(15):1224-1235. doi: 10.1093/ajhp/zxac105. Am J Health Syst Pharm. 2022. PMID: 35390118 Review.
Cited by
-
Androgen Plays a Potential Novel Hormonal Therapeutic Role in Th17 Cells Predominant Neutrophilic Severe Asthma by Attenuating BECs Regulated Th17 Cells Differentiation via MBD2 Expression.Oxid Med Cell Longev. 2022 Aug 25;2022:3096528. doi: 10.1155/2022/3096528. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36062195 Free PMC article.
-
Revisiting the roles of cAMP signalling in the progression of prostate cancer.Biochem J. 2023 Oct 31;480(20):1599-1614. doi: 10.1042/BCJ20230297. Biochem J. 2023. PMID: 37830741 Free PMC article.
-
Structures of the ADGRG2-Gs complex in apo and ligand-bound forms.Nat Chem Biol. 2022 Nov;18(11):1196-1203. doi: 10.1038/s41589-022-01084-6. Epub 2022 Aug 18. Nat Chem Biol. 2022. PMID: 35982227
-
G protein selectivity profile of GPR56/ADGRG1 and its effect on downstream effectors.Res Sq [Preprint]. 2024 Sep 5:rs.3.rs-4869264. doi: 10.21203/rs.3.rs-4869264/v1. Res Sq. 2024. Update in: Cell Mol Life Sci. 2024 Sep 4;81(1):383. doi: 10.1007/s00018-024-05416-8 PMID: 39281883 Free PMC article. Updated. Preprint.
-
Ablation of GPR56 Causes β-Cell Dysfunction by ATP Loss through Mistargeting of Mitochondrial VDAC1 to the Plasma Membrane.Biomolecules. 2023 Mar 18;13(3):557. doi: 10.3390/biom13030557. Biomolecules. 2023. PMID: 36979492 Free PMC article.
References
-
- Huggins Charles and Hodges Clarence V. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Research 1941; 1(4). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

