Efficacy Evaluation of Early, Low-Dose, Short-Term Corticosteroids in Adults Hospitalized with Non-Severe COVID-19 Pneumonia: A Retrospective Cohort Study
- PMID: 32880102
- PMCID: PMC7467137
- DOI: 10.1007/s40121-020-00332-3
Efficacy Evaluation of Early, Low-Dose, Short-Term Corticosteroids in Adults Hospitalized with Non-Severe COVID-19 Pneumonia: A Retrospective Cohort Study
Abstract
Objectives: This study aimed to observe the efficacy of corticosteroids in non-severe COVID-19 pneumonia.
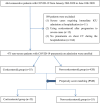
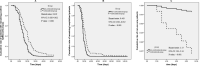
Methods: A retrospective study based on propensity score matching was designed to explore the effects of corticosteroids. Primary outcomes included the rate of patients who developed severe disease and mortality. Secondary outcomes included duration of fever, virus clearance time, length of hospital stay, and the use of antibiotics.
Results: A total of 475 patients with non-severe COVID-19 pneumonia were enrolled, 55 patients received early, low-dose, and short-term corticosteroids therapy, 420 patients received non-corticosteroids therapy. Compared to the non-corticosteroids group, there was a prolonged duration of fever (median 5 vs 3 days, p < 0.001), virus clearance time (median 18 vs 11 days, p < 0.001), and length of hospital stay (median 23 vs 15 days, p < 0.001) in the corticosteroids group. The percentages of antibiotics therapy (89.1% vs 23.6%, p < 0.001), use of at least two antibiotics (38.2% vs 12.7%, p = 0.002), and antifungal therapy (7.3% vs 0, p = 0.042) were higher in the corticosteroids group than those in the non-corticosteroids group. Compared to the non-corticosteroids group, more patients developed severe disease (12.7% vs 1.8%, p = 0.028) in the corticosteroids group. There was no significant difference between the two groups in mortality (1.8% vs 0, p = 0.315).
Conclusion: In adult patients with non-severe COVID-19 pneumonia, early, low-dose, and short-term corticosteroids therapy was associated with worse clinical outcomes.
Keywords: 2019 novel coronavirus disease; Corticosteroids; Efficacy evaluation; Non-severe COVID-19 infections; Severe acute respiratory syndrome coronavirus 2.
Figures
Similar articles
-
Efficacy evaluation of intravenous immunoglobulin in non-severe patients with COVID-19: A retrospective cohort study based on propensity score matching.Int J Infect Dis. 2021 Apr;105:525-531. doi: 10.1016/j.ijid.2021.01.009. Epub 2021 Jan 9. Int J Infect Dis. 2021. PMID: 33434674 Free PMC article.
-
Clinical Use of Short-Course and Low-Dose Corticosteroids in Patients With Non-severe COVID-19 During Pneumonia Progression.Front Public Health. 2020 Jul 3;8:355. doi: 10.3389/fpubh.2020.00355. eCollection 2020. Front Public Health. 2020. PMID: 32719766 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome.Cochrane Database Syst Rev. 2022 Mar 11;3(3):CD013130. doi: 10.1002/14651858.CD013130.pub2. Cochrane Database Syst Rev. 2022. PMID: 35274741 Free PMC article. Review.
-
Corticosteroid administration for viral pneumonia: COVID-19 and beyond.Clin Microbiol Infect. 2020 Sep;26(9):1171-1177. doi: 10.1016/j.cmi.2020.06.020. Epub 2020 Jun 27. Clin Microbiol Infect. 2020. PMID: 32603802 Free PMC article. Review.
Cited by
-
A "Window of Therapeutic Opportunity" for Anti-Cytokine Therapy in Patients With Coronavirus Disease 2019.Front Immunol. 2020 Oct 6;11:572635. doi: 10.3389/fimmu.2020.572635. eCollection 2020. Front Immunol. 2020. PMID: 33123149 Free PMC article.
-
Corticosteroids for non-severe COVID-19 infections? Too early to conclude.Korean J Intern Med. 2023 Mar;38(2):144-146. doi: 10.3904/kjim.2023.046. Epub 2023 Feb 27. Korean J Intern Med. 2023. PMID: 36864598 Free PMC article. No abstract available.
-
The Influence of Corticosteroids, Immunosuppressants and Biologics on Patients With Inflammatory Bowel Diseases, Psoriasis and Rheumatic Diseases in the Era of COVID-19: A Review of Current Evidence.Front Immunol. 2021 Jul 8;12:677957. doi: 10.3389/fimmu.2021.677957. eCollection 2021. Front Immunol. 2021. PMID: 34335579 Free PMC article. Review.
-
Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes.Crit Care. 2020 Dec 14;24(1):696. doi: 10.1186/s13054-020-03400-9. Crit Care. 2020. PMID: 33317589 Free PMC article.
-
Microbiological and Clinical Findings of SARS-CoV-2 Infection after 2 Years of Pandemic: From Lung to Gut Microbiota.Diagnostics (Basel). 2022 Sep 2;12(9):2143. doi: 10.3390/diagnostics12092143. Diagnostics (Basel). 2022. PMID: 36140544 Free PMC article. Review.
References
-
- World Health Organization. Coronavirus disease situation reports-166. 2019. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 4 Jul 2020.
Grants and funding
LinkOut - more resources
Full Text Sources

