Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker
- PMID: 32875727
- PMCID: PMC7643687
- DOI: 10.1002/cam4.3410
Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker
Abstract
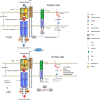
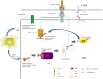
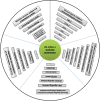
Immunocheckpoint proteins of tumor infiltrating lymphocytes play an important role in tumor prognosis in the course of tumor clinicopathology. PD-1 (Programmed cell death protein 1) is an important immunosuppressive molecule. By binding to PD-L1 (programmed cell death-ligand 1), it blocks TCR and its costimulus signal transduction, inhibits the activation and proliferation of T cells, depletes the function of effector T cells, and enables tumor cells to achieve immune escape. In recent years, immunocheckpoint blocking therapy targeting the PD-1/PD-L1 axis has achieved good results in a variety of malignant tumors, pushing tumor immunotherapy to a new milestone, such as anti-PD-1 monoclonal antibody Nivolumab, Pembrolizumab, and anti-PD-L1 monoclonal antibody Atezolizumab, which are considered as potential antitumor drugs. It was found in clinical use that some patients obtained long-term efficacy, but most of them developed drug resistance recurrence in the later stage. The high incidence of drug resistance (including primary and acquired drug resistance) still cannot be ignored, which limited its clinical application and became a new problem in this field. Due to tumor heterogeneity, current limited research shows that PD-1 or PD-L1 monoclonal antibody drug resistance may be related to the following factors: mutation of tumor antigen and antigen presentation process, multiple immune checkpoint interactions, immune microenvironment changes dynamically, activation of oncogenic pathways, gene mutation and epigenetic changes of key proteins in tumors, tumor competitive metabolism, and accumulation of metabolites, etc, mechanisms of resistance are complex. Therefore, it is the most urgent task to further elucidate the mechanism of immune checkpoint inhibitor resistance, discover multitumor universal biomarkers, and develop new target agents to improve the response rate of immunotherapy in patients. In this study, the mechanism of anti-PD-1/PD-L1 drug resistance in tumors, the potential biomarkers for predicting PD-1 acquired resistance, and the recent development of combination therapy were reviewed one by one. It is believed that, based on the complex mechanism of drug resistance, it is of no clinical significance to simply search for and regulate drug resistance targets, and it may even produce drug resistance again soon. It is speculated that according to the possible tumor characteristics, three types of treatment methods should be combined to change the tumor microenvironment ecology and eliminate various heterogeneous tumor subsets, so as to reduce tumor drug resistance and improve long-term clinical efficacy.
Keywords: PD-1/PD-L1; immunocheckpoint blocker; mechanism; resistance; study and analysis.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors Xiaoying Wu and Zhengyi Wang declare that they have no competing interests.
Figures
Similar articles
-
A Small Molecule Antagonist of PD-1/PD-L1 Interactions Acts as an Immune Checkpoint Inhibitor for NSCLC and Melanoma Immunotherapy.Front Immunol. 2021 May 14;12:654463. doi: 10.3389/fimmu.2021.654463. eCollection 2021. Front Immunol. 2021. PMID: 34054817 Free PMC article.
-
Research progress of PD-1/PD-L1 immunotherapy in gastrointestinal tumors.Biomed Pharmacother. 2020 Sep;129:110504. doi: 10.1016/j.biopha.2020.110504. Epub 2020 Jul 8. Biomed Pharmacother. 2020. PMID: 32768978 Review.
-
The recent advances of PD-1 and PD-L1 checkpoint signaling inhibition for breast cancer immunotherapy.Eur J Pharmacol. 2021 Mar 15;895:173867. doi: 10.1016/j.ejphar.2021.173867. Epub 2021 Jan 15. Eur J Pharmacol. 2021. PMID: 33460617 Review.
-
Tumor-derived exosomes in the PD-1/PD-L1 axis: Significant regulators as well as promising clinical targets.J Cell Physiol. 2021 Jun;236(6):4138-4151. doi: 10.1002/jcp.30197. Epub 2020 Dec 4. J Cell Physiol. 2021. PMID: 33275291 Review.
-
Potential and unsolved problems of anti-PD-1/PD-L1 therapy combined with radiotherapy.Tumori. 2021 Aug;107(4):282-291. doi: 10.1177/0300891620940382. Epub 2020 Jul 31. Tumori. 2021. PMID: 32734832 Review.
Cited by
-
Heterogeneity induced GZMA-F2R communication inefficient impairs antitumor immunotherapy of PD-1 mAb through JAK2/STAT1 signal suppression in hepatocellular carcinoma.Cell Death Dis. 2022 Mar 7;13(3):213. doi: 10.1038/s41419-022-04654-7. Cell Death Dis. 2022. PMID: 35256589 Free PMC article.
-
The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers.Front Immunol. 2022 Sep 13;13:964442. doi: 10.3389/fimmu.2022.964442. eCollection 2022. Front Immunol. 2022. PMID: 36177034 Free PMC article. Review.
-
The roles of KLHL family members in human cancers.Am J Cancer Res. 2022 Nov 15;12(11):5105-5139. eCollection 2022. Am J Cancer Res. 2022. PMID: 36504893 Free PMC article. Review.
-
Role of Ezrin/Radixin/Moesin in the Surface Localization of Programmed Cell Death Ligand-1 in Human Colon Adenocarcinoma LS180 Cells.Pharmaceuticals (Basel). 2021 Aug 28;14(9):864. doi: 10.3390/ph14090864. Pharmaceuticals (Basel). 2021. PMID: 34577564 Free PMC article.
-
Infiltrating treg reprogramming in the tumor immune microenvironment and its optimization for immunotherapy.Biomark Res. 2024 Sep 4;12(1):97. doi: 10.1186/s40364-024-00630-9. Biomark Res. 2024. PMID: 39227959 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

