AKT2 drives cancer progression and is negatively modulated by miR-124 in human lung adenocarcinoma
- PMID: 32873299
- PMCID: PMC7466426
- DOI: 10.1186/s12931-020-01491-0
AKT2 drives cancer progression and is negatively modulated by miR-124 in human lung adenocarcinoma
Abstract
Background: AKT2 is highly expressed in many human cancers, including non-small cell lung cancer (NSCLC). Accumulating evidence has also revealed that AKT2 can promote NSCLC cell proliferation and metastasis. However, the involved mechanism remains unclear. Herein, our study mainly explored the function of AKT2 during cancer progression and uncovered a new post-transcriptional mechanism of AKT2 expression in lung adenocarcinoma (LUAD).
Methods: Quantitative real-time (qRT-PCR), western blot and immunohistochemistry (IHC) assays were performed to detect the expression of AKT2 and other proteins. Cell counting kit-8 (CCK-8), colony formation and EdU assays were performed to assess cell proliferation. Flow cytometry analysis was used to detect changes in the cell cycle and apoptosis. Transwell assays were used to evaluate cell migration and invasion. Additionally, a luciferase reporter assay and western blotting were employed to assess miR-124 targeting of AKT2. Xenograft mouse model was used to observe the role of miR-124/AKT2 axis on the occurrence and development of LUAD.
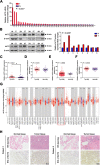
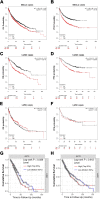
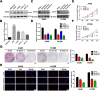
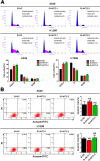
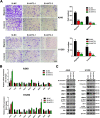
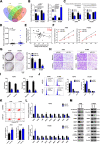
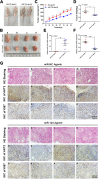
Results: We showed that AKT2 was highly expressed in NSCLC tissues and closely related to the poor prognosis of LUAD patients. Moreover, AKT2 affected LUAD cell proliferation, migration and invasion by regulating the cell cycle and promoting the occurrence of epithelial-mesenchymal transition (EMT) and the expression of matrix metalloproteinases (MMPs). In addition, we demonstrated that miR-124 overexpression downregulated AKT2 expression by binding to the 3'-untranslated region (3'- UTR) of AKT2 and thus inhibited the occurrence and development of LUAD in vivo and in vitro.
Conclusions: Our results suggest that miR-124 overexpression can negatively regulate AKT2 and thus inhibit the progression of LUAD. Therefore, the miR-124/AKT2 axis may serve as a potential target for novel therapies for LUAD.
Keywords: AKT2; Invasion; Lung adenocarcinoma; Migration; Proliferation; miR-124.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Long noncoding RNA CRNDE promotes non-small cell lung cancer progression via sponging microRNA-338-3p.Biomed Pharmacother. 2019 Feb;110:825-833. doi: 10.1016/j.biopha.2018.12.024. Epub 2018 Dec 14. Biomed Pharmacother. 2019. PMID: 30554121
-
MicroRNA-92a promotes epithelial-mesenchymal transition through activation of PTEN/PI3K/AKT signaling pathway in non-small cell lung cancer metastasis.Int J Oncol. 2017 Jul;51(1):235-244. doi: 10.3892/ijo.2017.3999. Epub 2017 May 16. Int J Oncol. 2017. PMID: 28534966
-
miR-15b enhances the proliferation and migration of lung adenocarcinoma by targeting BCL2.Thorac Cancer. 2020 Jun;11(6):1396-1405. doi: 10.1111/1759-7714.13382. Epub 2020 Mar 27. Thorac Cancer. 2020. PMID: 32220063 Free PMC article.
-
Long noncoding RNA SNHG7 contributes to cell proliferation, migration, invasion and epithelial to mesenchymal transition in non-small cell lung cancer by regulating miR-449a/TGIF2 axis.Thorac Cancer. 2020 Feb;11(2):264-276. doi: 10.1111/1759-7714.13245. Epub 2019 Dec 3. Thorac Cancer. 2020. PMID: 31793741 Free PMC article.
-
Circ_0074027 contributes to the progression of non-small cell lung cancer via microRNA-362-3p/clathrin heavy chain axis.Anticancer Drugs. 2021 Jan 1;32(1):1-10. doi: 10.1097/CAD.0000000000000990. Anticancer Drugs. 2021. PMID: 32932275
Cited by
-
Roles of microRNA-124 in traumatic brain injury: a comprehensive review.Front Cell Neurosci. 2023 Nov 28;17:1298508. doi: 10.3389/fncel.2023.1298508. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38034588 Free PMC article. Review.
-
Propofol mediates non-small cell lung cancer growth in part by regulating circ_0003028-related mechanisms.Thorac Cancer. 2023 Jun;14(17):1606-1617. doi: 10.1111/1759-7714.14906. Epub 2023 Apr 27. Thorac Cancer. 2023. PMID: 37105940 Free PMC article.
-
In vitro anticancer evaluation of Enceleamycin A and its underlying mechanism.RSC Adv. 2023 Nov 22;13(48):34183-34193. doi: 10.1039/d3ra06204j. eCollection 2023 Nov 16. RSC Adv. 2023. PMID: 38019992 Free PMC article.
-
Targeting TYK2 alleviates Rab27A-induced malignant progression of non-small cell lung cancer via disrupting IFNα-TYK2-STAT-HSPA5 axis.NPJ Precis Oncol. 2024 Mar 23;8(1):74. doi: 10.1038/s41698-024-00574-1. NPJ Precis Oncol. 2024. PMID: 38521810 Free PMC article.
-
The Antitumour Mechanisms of Carotenoids: A Comprehensive Review.Antioxidants (Basel). 2024 Aug 30;13(9):1060. doi: 10.3390/antiox13091060. Antioxidants (Basel). 2024. PMID: 39334719 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- No. 81802885/National Natural Science Foundation of China
- No. KJXW2016003/the Foundation of Health Care Rejuvenation by Science and Education
- No. SS201630/the Societal and Developmental Project of Suzhou
- No. SZS201617/the Suzhou Key Laboratory for Respiratory Medicine
- No.Szzx201502/the Clinical Medical Center of Suzhou
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

