Extracellular Neuroglobin as a Stress-Induced Factor Activating Pre-Adaptation Mechanisms against Oxidative Stress and Chemotherapy-Induced Cell Death in Breast Cancer
- PMID: 32872414
- PMCID: PMC7564643
- DOI: 10.3390/cancers12092451
Extracellular Neuroglobin as a Stress-Induced Factor Activating Pre-Adaptation Mechanisms against Oxidative Stress and Chemotherapy-Induced Cell Death in Breast Cancer
Abstract
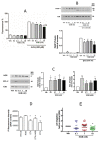
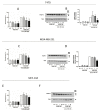
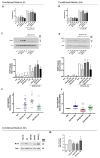
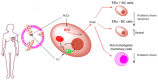
Components of tumor microenvironment, including tumor and/or stromal cells-derived factors, exert a critical role in breast cancer (BC) progression. Here we evaluated the possible role of neuroglobin (NGB), a monomeric globin that acts as a compensatory protein against oxidative and apoptotic processes, as part of BC microenvironment. The extracellular NGB levels were evaluated by immunofluorescence of BC tissue sections and by Western blot of the culture media of BC cell lines. Moreover, reactive oxygen species (ROS) generation, cell apoptosis, and cell migration were evaluated in different BC cells and non-tumorigenic epithelial mammary cells treated with BC cells (i.e., Michigan Cancer Foundation-7, MCF-7) conditioned culture media and extracellular NGB. Results demonstrate that NGB is a component of BC microenvironment. NGB is released in tumor microenvironment by BC cells only under oxidative stress conditions where it can act as autocrine/paracrine factor able to communicate cell resilience against oxidative stress and chemotherapeutic treatment.
Keywords: 17β-Estradiol; apoptosis; breast cancer; docetaxel; neuroglobin; oxidative stress; stress adaptation and resistance; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Neuroglobin As Key Mediator in the 17β-Estradiol-Induced Antioxidant Cell Response to Oxidative Stress.Antioxid Redox Signal. 2020 Feb 1;32(4):217-227. doi: 10.1089/ars.2019.7870. Antioxid Redox Signal. 2020. PMID: 31686530
-
Dissecting the 17β-estradiol pathways necessary for neuroglobin anti-apoptotic activity in breast cancer.J Cell Physiol. 2018 Jul;233(7):5087-5103. doi: 10.1002/jcp.26378. Epub 2018 Jan 19. J Cell Physiol. 2018. PMID: 29219195
-
Neuroglobin in Breast Cancer Cells: Effect of Hypoxia and Oxidative Stress on Protein Level, Localization, and Anti-Apoptotic Function.PLoS One. 2016 May 5;11(5):e0154959. doi: 10.1371/journal.pone.0154959. eCollection 2016. PLoS One. 2016. PMID: 27149623 Free PMC article.
-
Neuroglobin: A Novel Player in the Oxidative Stress Response of Cancer Cells.Oxid Med Cell Longev. 2019 Jul 1;2019:6315034. doi: 10.1155/2019/6315034. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31354909 Free PMC article. Review.
-
Neuroglobin and neuronal cell survival.Biochim Biophys Acta. 2013 Sep;1834(9):1744-9. doi: 10.1016/j.bbapap.2013.01.015. Epub 2013 Jan 26. Biochim Biophys Acta. 2013. PMID: 23357651 Review.
Cited by
-
Overexpression of Neuroglobin Promotes Energy Metabolism and Autophagy Induction in Human Neuroblastoma SH-SY5Y Cells.Cells. 2021 Dec 2;10(12):3394. doi: 10.3390/cells10123394. Cells. 2021. PMID: 34943907 Free PMC article.
-
Peroxidase activity of rice (Oryza sativa) hemoglobin: distinct role of tyrosines 112 and 151.J Biol Inorg Chem. 2023 Sep;28(6):613-626. doi: 10.1007/s00775-023-02014-0. Epub 2023 Jul 29. J Biol Inorg Chem. 2023. PMID: 37507628
-
Neuroglobin in Retinal Neurodegeneration: A Potential Target in Therapeutic Approaches.Cells. 2021 Nov 17;10(11):3200. doi: 10.3390/cells10113200. Cells. 2021. PMID: 34831423 Free PMC article. Review.
-
Neuroglobin: A New Possible Marker of Estrogen-Responsive Breast Cancer.Cells. 2021 Aug 5;10(8):1986. doi: 10.3390/cells10081986. Cells. 2021. PMID: 34440755 Free PMC article.
-
Proteomic and Bioinformatic Investigation of Altered Pathways in Neuroglobin-Deficient Breast Cancer Cells.Molecules. 2021 Apr 20;26(8):2397. doi: 10.3390/molecules26082397. Molecules. 2021. PMID: 33924212 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

