Flu Universal Vaccines: New Tricks on an Old Virus
- PMID: 32870450
- PMCID: PMC7459973
- DOI: 10.1007/s12250-020-00283-6
Flu Universal Vaccines: New Tricks on an Old Virus
Abstract
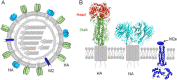
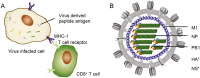
Conventional influenza vaccines are based on predicting the circulating viruses year by year, conferring limited effectiveness since the antigenicity of vaccine strains does not always match the circulating viruses. This necessitates development of universal influenza vaccines that provide broader and lasting protection against pan-influenza viruses. The discovery of the highly conserved immunogens (epitopes) of influenza viruses provides attractive targets for universal vaccine design. Here we review the current understanding with broadly protective immunogens (epitopes) and discuss several important considerations to achieve the goal of universal influenza vaccines.
Keywords: Broadly protection; Conserved epitopes; Influenza virus; Universal vaccine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Immunodominance and Antigenic Variation of Influenza Virus Hemagglutinin: Implications for Design of Universal Vaccine Immunogens.J Infect Dis. 2019 Apr 8;219(Suppl_1):S38-S45. doi: 10.1093/infdis/jiy696. J Infect Dis. 2019. PMID: 30535315 Free PMC article.
-
The Quest for a Universal Flu Vaccine: Headless HA 2.0.Cell Host Microbe. 2015 Oct 14;18(4):395-7. doi: 10.1016/j.chom.2015.10.003. Cell Host Microbe. 2015. PMID: 26468743
-
Mapping of a Novel H3-Specific Broadly Neutralizing Monoclonal Antibody Targeting the Hemagglutinin Globular Head Isolated from an Elite Influenza Virus-Immunized Donor Exhibiting Serological Breadth.J Virol. 2020 Feb 28;94(6):e01035-19. doi: 10.1128/JVI.01035-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31826999 Free PMC article.
-
Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine.Viruses. 2021 Mar 24;13(4):546. doi: 10.3390/v13040546. Viruses. 2021. PMID: 33805245 Free PMC article. Review.
-
Recent Advances, Approaches and Challenges in the Development of Universal Influenza Vaccines.Influenza Other Respir Viruses. 2024 Mar;18(3):e13276. doi: 10.1111/irv.13276. Influenza Other Respir Viruses. 2024. PMID: 38513364 Free PMC article. Review.
Cited by
-
Optimization and applications of an in vivo bioluminescence imaging model of influenza A virus infections.Virol Sin. 2023 Aug;38(4):631-634. doi: 10.1016/j.virs.2023.04.007. Epub 2023 May 2. Virol Sin. 2023. PMID: 37141991 Free PMC article.
-
Single-Component Multilayered Self-Assembling Protein Nanoparticles Displaying Extracellular Domains of Matrix Protein 2 as a Pan-influenza A Vaccine.ACS Nano. 2023 Dec 12;17(23):23545-23567. doi: 10.1021/acsnano.3c06526. Epub 2023 Nov 21. ACS Nano. 2023. PMID: 37988765 Free PMC article.
-
Influenza Virus-Derived CD8 T Cell Epitopes: Implications for the Development of Universal Influenza Vaccines.Immune Netw. 2024 May 7;24(3):e19. doi: 10.4110/in.2024.24.e19. eCollection 2024 Jun. Immune Netw. 2024. PMID: 38974213 Free PMC article. Review.
-
Revisiting influenza A virus life cycle from a perspective of genome balance.Virol Sin. 2023 Feb;38(1):1-8. doi: 10.1016/j.virs.2022.10.005. Epub 2022 Oct 27. Virol Sin. 2023. PMID: 36309307 Free PMC article. Review.
-
SARS-CoV-2 cell entry and targeted antiviral development.Acta Pharm Sin B. 2021 Dec;11(12):3879-3888. doi: 10.1016/j.apsb.2021.05.007. Epub 2021 May 13. Acta Pharm Sin B. 2021. PMID: 34002130 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

