Propofol inhibits the expression of Abelson nonreceptor tyrosine kinase without affecting learning or memory function in neonatal rats
- PMID: 32869521
- PMCID: PMC7667295
- DOI: 10.1002/brb3.1810
Propofol inhibits the expression of Abelson nonreceptor tyrosine kinase without affecting learning or memory function in neonatal rats
Abstract
Objective: Propofol is one of the most commonly used intravenous drugs to induce and maintain general anesthesia. In vivo and in vitro studies have shown that propofol can affect neuronal growth, leading to apoptosis and impairing cognitive function. The Abelson nonreceptor tyrosine kinase (c-Abl) is associated with both neuritic plaques and neurofibrillary tangles in the brains of patients with Alzheimer's disease and other neurodegenerative diseases. This study aimed to explore the effect of propofol on apoptosis and neurocognition through its regulation of c-Abl expression in vivo and in vitro.
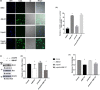
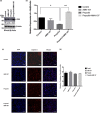
Materials and methods: In this study, primary hippocampal neurons were cultured and exposed to propofol at different concentrations. Protein expression was measured by Western blotting and coimmunoprecipitation. The c-Abl transcription level was verified by fluorescence quantitative PCR. Reactive oxygen species (ROS) levels were detected by flow cytometry. In addition, an animal experiment was conducted to assess neuronal apoptosis by immunofluorescence staining for caspase-3 and to evaluate behavioral changes by the Morris water maze (MWM) test.
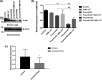
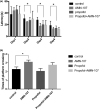
Results: The in vitro experiment showed that propofol significantly decreased c-Abl expression and ROS levels. In addition, propofol has no cytotoxic effect and does not affect cell activity. Moreover, in the animal experiment, intraperitoneal injection of 50 mg/kg propofol for 5 days obviously decreased the expression of c-Abl in the neonatal rat brain (p < .05) but did not significantly increase the number of caspase-3-positive cells. Propofol treatment did not significantly reduce the number of platform crossings (p > .05) or prolong the escape latency of neonatal rats (p > .05) in the MWM test.
Conclusions: The present data suggest that reduced expression of this nonreceptor tyrosine kinase through consecutive daily administration of propofol did not impair learning or memory function in neonatal rats.
Keywords: ROS; abelson nonreceptor tyrosine kinase; apoptosis; neonatal rat; neurocognitive dysfunction; propofol.
© 2020 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Repeated exposure to propofol in the neonatal period impairs hippocampal synaptic plasticity and the recognition function of rats in adulthood.Brain Res Bull. 2021 Apr;169:63-72. doi: 10.1016/j.brainresbull.2021.01.007. Epub 2021 Jan 13. Brain Res Bull. 2021. PMID: 33450329
-
[Influences of repeated propofol anesthesia on hippocampal apoptosis and long-term learning and memory abilities of neonatal rats].Beijing Da Xue Xue Bao Yi Xue Ban. 2017 Apr 18;49(2):310-314. Beijing Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28416843 Chinese.
-
[Effects of propofol on expression of hippocampal survivin and Caspase-3 in newborn rats].Zhonghua Er Ke Za Zhi. 2012 May;50(5):361-5. Zhonghua Er Ke Za Zhi. 2012. PMID: 22883038 Chinese.
-
Hippocampal SIRT1-Mediated Synaptic Plasticity and Glutamatergic Neuronal Excitability Are Involved in Prolonged Cognitive Dysfunction of Neonatal Rats Exposed to Propofol.Mol Neurobiol. 2022 Mar;59(3):1938-1953. doi: 10.1007/s12035-021-02684-4. Epub 2022 Jan 16. Mol Neurobiol. 2022. PMID: 35034265
-
Propofol-induced hippocampal Neurotoxicity: A mitochondrial perspective.Brain Res. 2024 May 15;1831:148841. doi: 10.1016/j.brainres.2024.148841. Epub 2024 Feb 29. Brain Res. 2024. PMID: 38428475 Review.
Cited by
-
An Integrative Bioinformatics Analysis of the Potential Mechanisms Involved in Propofol Affecting Hippocampal Neuronal Cells.Comput Intell Neurosci. 2022 Apr 26;2022:4911773. doi: 10.1155/2022/4911773. eCollection 2022. Comput Intell Neurosci. 2022. PMID: 35515499 Free PMC article.
-
Roles for c-Abl in postoperative neurodegeneration.Int J Med Sci. 2022 Sep 28;19(12):1753-1761. doi: 10.7150/ijms.73740. eCollection 2022. Int J Med Sci. 2022. PMID: 36313229 Free PMC article. Review.
-
Dihydroartemisinin Inhibits TGF-β-Induced Fibrosis in Human Tenon Fibroblasts via Inducing Autophagy.Drug Des Devel Ther. 2021 Mar 3;15:973-981. doi: 10.2147/DDDT.S280322. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33688170 Free PMC article.
-
The Role of c-Abl Tyrosine Kinase in Brain and Its Pathologies.Cells. 2023 Aug 10;12(16):2041. doi: 10.3390/cells12162041. Cells. 2023. PMID: 37626851 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

