Kaposi's Sarcoma-Associated Herpesvirus Reactivation by Targeting of a dCas9-Based Transcription Activator to the ORF50 Promoter
- PMID: 32867368
- PMCID: PMC7552072
- DOI: 10.3390/v12090952
Kaposi's Sarcoma-Associated Herpesvirus Reactivation by Targeting of a dCas9-Based Transcription Activator to the ORF50 Promoter
Abstract
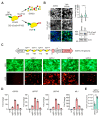
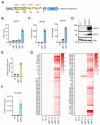
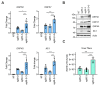
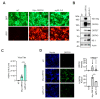
CRISPR activation (CRISPRa) has revealed great potential as a tool to modulate the expression of targeted cellular genes. Here, we successfully applied the CRISPRa system to trigger the Kaposi's sarcoma-associated herpesvirus (KSHV) reactivation in latently infected cells by selectively activating ORF50 gene directly from the virus genome. We found that a nuclease-deficient Cas9 (dCas9) fused to a destabilization domain (DD) and 12 copies of the VP16 activation domain (VP192) triggered a more efficient KSHV lytic cycle and virus production when guided to two different sites on the ORF50 promoter, instead of only a single site. To our surprise, the virus reactivation induced by binding of the stable DD-dCas9-VP192 on the ORF50 promoter was even more efficient than reactivation induced by ectopic expression of ORF50. This suggests that recruitment of additional transcriptional activators to the ORF50 promoter, in addition to ORF50 itself, are needed for the efficient virus production. Further, we show that CRISPRa can be applied to selectively express the early lytic gene, ORF57, without disturbing the viral latency. Therefore, CRISPRa-based systems can be utilized to facilitate virus-host interaction studies by controlling the expression of not only cellular but also of specific KSHV genes.
Keywords: CRISPRa; DD-dCas9-VP192; KSHV; KSHV lytic cycle; KSHV reactivation; Kaposi’s sarcoma-associated herpesvirus; ORF50; RTA; dCas9.
Conflict of interest statement
We declare no conflict of interest.
Figures
Similar articles
-
Identification of Novel Kaposi's Sarcoma-Associated Herpesvirus Orf50 Transcripts: Discovery of New RTA Isoforms with Variable Transactivation Potential.J Virol. 2016 Dec 16;91(1):e01434-16. doi: 10.1128/JVI.01434-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795414 Free PMC article.
-
Effects of the NEDD8-Activating Enzyme Inhibitor MLN4924 on Lytic Reactivation of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2017 Sep 12;91(19):e00505-17. doi: 10.1128/JVI.00505-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701396 Free PMC article.
-
Chromatin remodeling of the Kaposi's sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency.J Virol. 2003 Nov;77(21):11425-35. doi: 10.1128/jvi.77.21.11425-11435.2003. J Virol. 2003. PMID: 14557628 Free PMC article.
-
The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review.
-
Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis.Microbiol Mol Biol Rev. 2003 Jun;67(2):175-212, table of contents. doi: 10.1128/MMBR.67.2.175-212.2003. Microbiol Mol Biol Rev. 2003. PMID: 12794189 Free PMC article. Review.
Cited by
-
CRISPR-Mediated Gene Activation (CRISPRa) of pp38/pp24 Orchestrates Events Triggering Lytic Infection in Marek's Disease Virus-Transformed Cell Lines.Microorganisms. 2021 Aug 8;9(8):1681. doi: 10.3390/microorganisms9081681. Microorganisms. 2021. PMID: 34442761 Free PMC article.
-
Targeted eradication of EBV-positive cancer cells by CRISPR/dCas9-mediated EBV reactivation in combination with ganciclovir.mBio. 2024 Jul 17;15(7):e0079524. doi: 10.1128/mbio.00795-24. Epub 2024 Jun 14. mBio. 2024. PMID: 38874417 Free PMC article.
-
Current Approaches to Epigenetic Therapy.Epigenomes. 2023 Sep 30;7(4):23. doi: 10.3390/epigenomes7040023. Epigenomes. 2023. PMID: 37873808 Free PMC article. Review.
-
CRISPR Interference Efficiently Silences Latent and Lytic Viral Genes in Kaposi's Sarcoma-Associated Herpesvirus-Infected Cells.Viruses. 2021 Apr 28;13(5):783. doi: 10.3390/v13050783. Viruses. 2021. PMID: 33924938 Free PMC article.
References
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d’Agay M.F., Clauvel J.P., Raphael M., Degos L., et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. doi: 10.1182/blood.V86.4.1276.bloodjournal8641276. - DOI - PubMed
-
- Uldrick T.S., Wang V., O’Mahony D., Aleman K., Wyvill K.M., Marshall V., Steinberg S.M., Pittaluga S., Maric I., Whitby D., et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin. Infect. Dis. 2010;51:350–358. doi: 10.1086/654798. - DOI - PMC - PubMed
-
- Achenbach C.J., Harrington R.D., Dhanireddy S., Crane H.M., Casper C., Kitahata M.M. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin. Infect. Dis. 2012;54:424–433. doi: 10.1093/cid/cir802. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

