Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial
- PMID: 32862111
- PMCID: PMC7445008
- DOI: 10.1016/j.intimp.2020.106903
Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial
Abstract
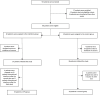
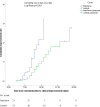
In this study, efficacy and safety of interferon (IFN) β-1b in the treatment of patients with severe COVID-19 were evaluated. Among an open-label, randomized clinical trial, adult patients (≥18 years old) with severe COVID-19 were randomly assigned (1:1) to the IFN group or the control group. Patients in the IFN group received IFN β-1b (250 mcg subcutaneously every other day for two consecutive weeks) along with the national protocol medications while in the control group, patients received only the national protocol medications (lopinavir/ritonavir or atazanavir/ritonavir plus hydroxychloroquine for 7-10 days). The primary outcome of the study was time to clinical improvement. Secondary outcomes were in-hospital complications and 28-daymortality. Between April 20 and May 20, 2020, 80 patients were enrolled and finally 33 patients in each group completed the study. Time to clinical improvment in the IFN group was significantly shorter than the control group ([9(6-10) vs. 11(9-15) days respectively, p = 0.002, HR = 2.30; 95% CI: 1.33-3.39]). At day 14, the percentage of discharged patients was 78.79% and 54.55% in the IFN and control groups respectively (OR = 3.09; 95% CI: 1.05-9.11, p = 0.03). ICU admission rate in the control group was significantly higher than the IFN group (66.66% vs. 42.42%, p = 0.04). The duration of hospitalization and ICU stay were not significantly different between the groups All-cause 28-day mortality was 6.06% and 18.18% in the IFN and control groups respectively (p = 0.12). IFN β-1b was effective in shortening the time to clinical improvement without serious adverse events in patients with severe COVID-19. Furthermore, admission in ICU and need for invasive mechanical ventilation decreased following administration of IFN β-1b. Although 28-day mortality was lower in the IFN group, further randomized clinical trials with large sample size are needed for exact estimation of survival benefit of IFN β-1b.
Keywords: COVID19; Interferon β; Iran; SARS-COV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
-
A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01061-20. doi: 10.1128/AAC.01061-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32661006 Free PMC article. Clinical Trial.
-
Evaluation of the efficacy and safety of favipiravir and interferon compared to lopinavir/ritonavir and interferon in moderately ill patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Oct 27;21(1):886. doi: 10.1186/s13063-020-04747-8. Trials. 2020. PMID: 33109246 Free PMC article.
-
Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial.Lancet. 2020 May 30;395(10238):1695-1704. doi: 10.1016/S0140-6736(20)31042-4. Epub 2020 May 10. Lancet. 2020. PMID: 32401715 Free PMC article. Clinical Trial.
-
Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Oct 20;21(1):866. doi: 10.1186/s13063-020-04774-5. Trials. 2020. PMID: 33081849 Free PMC article. Clinical Trial.
Cited by
-
The time to offer treatments for COVID-19.Expert Opin Investig Drugs. 2021 May;30(5):505-518. doi: 10.1080/13543784.2021.1901883. Epub 2021 Apr 23. Expert Opin Investig Drugs. 2021. PMID: 33721548 Free PMC article. Review.
-
In silico screening and evaluation of antiviral peptides as inhibitors against ORF9b protein of SARS-CoV-2.3 Biotech. 2024 Sep;14(9):192. doi: 10.1007/s13205-024-04032-4. Epub 2024 Aug 6. 3 Biotech. 2024. PMID: 39118822 Review.
-
Review: The Nose as a Route for Therapy. Part 2 Immunotherapy.Front Allergy. 2021 Jul 1;2:668781. doi: 10.3389/falgy.2021.668781. eCollection 2021. Front Allergy. 2021. PMID: 35387044 Free PMC article. Review.
-
Comparative effectiveness of pharmacological interventions on mortality and the average length of hospital stay of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials.Expert Rev Anti Infect Ther. 2022 Apr;20(4):585-609. doi: 10.1080/14787210.2022.1997587. Epub 2021 Dec 29. Expert Rev Anti Infect Ther. 2022. PMID: 34694949 Free PMC article.
-
Efficacy and safety of current treatment interventions for patients with severe COVID-19 infection: A network meta-analysis of randomized controlled trials.J Med Virol. 2022 Apr;94(4):1617-1626. doi: 10.1002/jmv.27512. Epub 2021 Dec 18. J Med Virol. 2022. PMID: 34882805 Free PMC article.
References
-
- Johns Hopkins Coronavirus Resource Center Home Page. July 5, 2020 (https://coronavirus.jhu.edu/map.html).
-
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., de Castilla D.L., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous