Immunogenicity of a single-dose compared with a two-dose primary series followed by a booster dose of ten-valent or 13-valent pneumococcal conjugate vaccine in South African children: an open-label, randomised, non-inferiority trial
- PMID: 32857992
- PMCID: PMC7689288
- DOI: 10.1016/S1473-3099(20)30289-9
Immunogenicity of a single-dose compared with a two-dose primary series followed by a booster dose of ten-valent or 13-valent pneumococcal conjugate vaccine in South African children: an open-label, randomised, non-inferiority trial
Erratum in
-
Correction to Lancet Infect Dis 2020; published online Aug 25. https://doi.org/10.1016/S1473-3099(20)30289-9.Lancet Infect Dis. 2020 Nov;20(11):e275. doi: 10.1016/S1473-3099(20)30741-6. Epub 2020 Sep 11. Lancet Infect Dis. 2020. PMID: 32926835 Free PMC article. No abstract available.
Abstract
Background: Routine childhood immunisation with pneumococcal conjugate vaccine (PCV) has changed the epidemiology of pneumococcal disease across age groups, providing an opportunity to reconsider PCV dosing schedules. We aimed to evaluate the post-booster dose immunogenicity of ten-valent (PCV10) and 13-valent (PCV13) PCVs between infants randomly assigned to receive a single-dose compared with a two-dose primary series.
Methods: We did an open-label, non-inferiority, randomised study in HIV-unexposed infants at a single centre in Soweto, South Africa. Infants were randomly assigned to receive one priming dose of PCV10 or PCV13 at ages 6 weeks (6w + 1 PCV10 and 6w + 1 PCV13 groups) or 14 weeks (14w + 1 PCV10 and 14w + 1 PCV13 groups) or two priming doses of PCV10 or PCV13, one each at ages 6 weeks and 14 weeks (2 + 1 PCV10 and 2 + 1 PCV13 groups); all participants then received a booster dose of PCV10 or PCV13 at 40 weeks of age. The primary endpoint was geometric mean concentrations (GMCs) of serotype-specific IgG 1 month after the booster dose, which was assessed in all participants who received PCV10 or PCV13 as per the assigned randomisation group and for whom laboratory results were available at that timepoint. The 1 + 1 vaccine schedule was considered non-inferior to the 2 + 1 vaccine schedule if the lower bound of the 96% CI for the GMC ratio was greater than 0·5 for at least ten PCV13 serotypes and eight PCV10 serotypes. Safety was a secondary endpoint. This trial is registered with ClinicalTrials.gov (NCT02943902) and is ongoing.
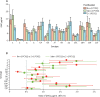
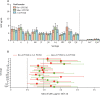
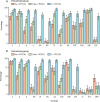
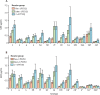
Findings: Of 1695 children assessed, 600 were enrolled and randomly assigned to one of the six groups between Jan 9 and Sept 20, 2017; 542 were included in the final analysis of the primary endpoint (86-93 per group). For both PCV13 and PCV10, a 1+1 dosing schedule (either beginning at 6 or 14 weeks) was non-inferior to a 2 + 1 schedule. For PCV13, the lower limit of the 96% CI for the ratio of GMCs between the 1 + 1 and 2 + 1 groups was higher than 0·5 for ten serotypes in the 6w+1 group (excluding 6B, 14, and 23F) and 11 serotypes in the 14w + 1 group (excluding 6B and 23F). For PCV10, the lower limit of the 96% CI for the ratio of GMCs was higher than 0·5 for all ten serotypes in the 6w+1 and 14w + 1 groups. 84 serious adverse events were reported in 72 (12%) of 600 participants. 15 occurred within 28 days of vaccination, but none were considered to be related to PCV injection. There were no cases of culture-confirmed invasive pneumococcal disease.
Interpretation: The non-inferiority in post-booster immune responses following a single-dose compared with a two-dose primary series of PCV13 or PCV10 indicates the potential for reducing PCV dosing schedules from a 2 + 1 to 1 + 1 series in low-income and middle-income settings with well established PCV immunisation programmes.
Funding: The Bill & Melinda Gates Foundation (OPP1 + 152352).
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Assessing reduced-dose pneumococcal vaccine schedules in South Africa.Lancet Infect Dis. 2020 Dec;20(12):1355-1357. doi: 10.1016/S1473-3099(20)30577-6. Epub 2020 Aug 25. Lancet Infect Dis. 2020. PMID: 32857991 No abstract available.
Similar articles
-
Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: a randomised controlled trial.Lancet Infect Dis. 2019 May;19(5):497-509. doi: 10.1016/S1473-3099(18)30734-5. Epub 2019 Apr 8. Lancet Infect Dis. 2019. PMID: 30975525 Free PMC article. Clinical Trial.
-
Single priming and booster dose of ten-valent and 13-valent pneumococcal conjugate vaccines and Streptococcus pneumoniae colonisation in children in South Africa: a single-centre, open-label, randomised trial.Lancet Child Adolesc Health. 2023 May;7(5):326-335. doi: 10.1016/S2352-4642(23)00025-1. Epub 2023 Mar 16. Lancet Child Adolesc Health. 2023. PMID: 36934731 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial.Lancet Infect Dis. 2021 Jun;21(6):834-846. doi: 10.1016/S1473-3099(20)30735-0. Epub 2021 Jan 28. Lancet Infect Dis. 2021. PMID: 33516293 Clinical Trial.
-
Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on immunogenicity.Pediatr Infect Dis J. 2014 Jan;33 Suppl 2(Suppl 2 Optimum Dosing of Pneumococcal Conjugate Vaccine For Infants 0 A Landscape Analysis of Evidence Supportin g Different Schedules):S119-29. doi: 10.1097/INF.0000000000000079. Pediatr Infect Dis J. 2014. PMID: 24336054 Free PMC article. Review.
-
Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy.Vaccine. 2012 Sep 7;30 Suppl 3:C21-7. doi: 10.1016/j.vaccine.2012.05.055. Vaccine. 2012. PMID: 22939016 Review.
Cited by
-
Vaccines to Prevent Meningitis: Historical Perspectives and Future Directions.Microorganisms. 2021 Apr 7;9(4):771. doi: 10.3390/microorganisms9040771. Microorganisms. 2021. PMID: 33917003 Free PMC article. Review.
-
The Divergent Effect of Different Infant Vaccination Schedules of the 13-Valent Pneumococcal Conjugate Vaccine on Serotype-Specific Immunological Memory.Vaccines (Basel). 2024 Sep 7;12(9):1024. doi: 10.3390/vaccines12091024. Vaccines (Basel). 2024. PMID: 39340054 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Hearing loss in Australian First Nations children at 6-monthly assessments from age 12 to 36 months: Secondary outcomes from randomised controlled trials of novel pneumococcal conjugate vaccine schedules.PLoS Med. 2024 Jun 3;21(6):e1004375. doi: 10.1371/journal.pmed.1004375. eCollection 2024 Jun. PLoS Med. 2024. PMID: 38829821 Free PMC article. Clinical Trial.
-
Efficacy against pneumococcal carriage and the immunogenicity of reduced-dose (0 + 1 and 1 + 1) PCV10 and PCV13 schedules in Ho Chi Minh City, Viet Nam: a parallel, single-blind, randomised controlled trial.Lancet Infect Dis. 2023 Aug;23(8):933-944. doi: 10.1016/S1473-3099(23)00061-0. Epub 2023 Apr 14. Lancet Infect Dis. 2023. PMID: 37062304 Free PMC article. Clinical Trial.
References
-
- WHO Pneumococcal vaccines WHO position paper—2012. Wkly Epidemiol Rec. 2012;87:129–144. - PubMed
-
- Whitney CG, Pilishvili T, Farley MM. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. - PubMed
-
- Jayasinghe S, Chiu C, Quinn H, Menzies R, Gilmour R, McIntyre P. Effectiveness of 7- and 13-valent pneumococcal conjugate vaccines in a schedule without a booster dose: a 10-year observational study. Clin Infect Dis. 2018;67:367–374. - PubMed
-
- Klugman KP, Madhi SA, Adegbola RA, Cutts F, Greenwood B, Hausdorff WP. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine. 2011;29:3372–3373. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

