In vitro and in vivo growth inhibitory activities of cryptolepine hydrate against several Babesia species and Theileria equi
- PMID: 32853247
- PMCID: PMC7451656
- DOI: 10.1371/journal.pntd.0008489
In vitro and in vivo growth inhibitory activities of cryptolepine hydrate against several Babesia species and Theileria equi
Abstract
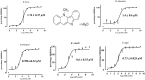
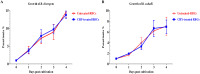
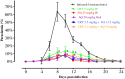
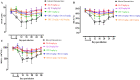
Piroplasmosis treatment has been based on the use of imidocarb dipropionate or diminazene aceturate (DA), however, their toxic effects. Therefore, the discovery of new drug molecules and targets is urgently needed. Cryptolepine (CRY) is a pharmacologically active plant alkaloid; it has significant potential as an antiprotozoal and antibacterial under different in vitro and in vivo conditions. The fluorescence assay was used for evaluating the inhibitory effect of CRY on four Babesia species and Theileria equi in vitro, and on the multiplication of B. microti in mice. The toxicity assay was evaluated on Madin-Darby bovine kidney (MDBK), mouse embryonic fibroblast (NIH/3T3), and human foreskin fibroblast (HFF) cell lines. The half-maximal inhibitory concentration (IC50) values of CRY on Babesia bovis, B. bigemina, B. divergens, B. caballi, and T. equi were 1740 ± 0.377, 1400 ± 0.6, 790 ± 0.32, 600 ± 0.53, and 730 ± 0.025 nM, respectively. The toxicity assay on MDBK, NIH/3T3, and HFF cell lines showed that CRY affected the viability of cells with a half-maximum effective concentration (EC50) of 86.67 ± 4.43, 95.29 ± 2.7, and higher than 100 μM, respectively. In mice experiments, CRY at a concentration of 5 mg/kg effectively inhibited the growth of B. microti, while CRY-atovaquone (AQ) and CRY-DA combinations showed higher chemotherapeutic effects than CRY alone. Our results showed that CRY has the potential to be an alternative remedy for treating piroplasmosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria.PLoS Negl Trop Dis. 2019 May 24;13(5):e0007030. doi: 10.1371/journal.pntd.0007030. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31125333 Free PMC article.
-
Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo.Parasit Vectors. 2019 May 28;12(1):269. doi: 10.1186/s13071-019-3520-x. Parasit Vectors. 2019. PMID: 31138282 Free PMC article.
-
17-DMAG inhibits the multiplication of several Babesia species and Theileria equi on in vitro cultures, and Babesia microti in mice.Int J Parasitol Drugs Drug Resist. 2018 Apr;8(1):104-111. doi: 10.1016/j.ijpddr.2018.02.005. Epub 2018 Mar 1. Int J Parasitol Drugs Drug Resist. 2018. PMID: 29499568 Free PMC article.
-
Discovering the in vitro potent inhibitors against Babesia and Theileria parasites by repurposing the Malaria Box: A review.Vet Parasitol. 2019 Oct;274:108895. doi: 10.1016/j.vetpar.2019.07.003. Epub 2019 Jul 19. Vet Parasitol. 2019. PMID: 31494399 Review.
-
Antiprotozoal treatment of canine babesiosis.Vet Parasitol. 2018 Apr 30;254:58-63. doi: 10.1016/j.vetpar.2018.03.001. Epub 2018 Mar 7. Vet Parasitol. 2018. PMID: 29657012 Review.
Cited by
-
Human Babesiosis in Europe.Pathogens. 2021 Sep 9;10(9):1165. doi: 10.3390/pathogens10091165. Pathogens. 2021. PMID: 34578196 Free PMC article. Review.
-
Cryptolepine Suppresses Colorectal Cancer Cell Proliferation, Stemness, and Metastatic Processes by Inhibiting WNT/β-Catenin Signaling.Pharmaceuticals (Basel). 2023 Jul 19;16(7):1026. doi: 10.3390/ph16071026. Pharmaceuticals (Basel). 2023. PMID: 37513937 Free PMC article.
-
Botanical Medicines Cryptolepis sanguinolenta, Artemisia annua, Scutellaria baicalensis, Polygonum cuspidatum, and Alchornea cordifolia Demonstrate Inhibitory Activity Against Babesia duncani.Front Cell Infect Microbiol. 2021 Mar 8;11:624745. doi: 10.3389/fcimb.2021.624745. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33763384 Free PMC article.
-
Harnessing Mycobacterium bovis BCG Trained Immunity to Control Human and Bovine Babesiosis.Vaccines (Basel). 2022 Jan 14;10(1):123. doi: 10.3390/vaccines10010123. Vaccines (Basel). 2022. PMID: 35062784 Free PMC article. Review.
-
Treatment of Human Babesiosis: Then and Now.Pathogens. 2021 Sep 1;10(9):1120. doi: 10.3390/pathogens10091120. Pathogens. 2021. PMID: 34578153 Free PMC article. Review.
References
-
- Batiha GE-S, Beshbishy AM, Guswanto A, Nugraha AB, Munkhjargal T, Abdel-Daim MM, et al. Phytochemical characterization and chemotherapeutic potential of Cinnamomum verum extracts on the multiplication of protozoan parasites in vitro and in vivo. Molecules. 2020a; 25(4): 996 10.3390/molecules25040996 - DOI - PMC - PubMed
-
- Hines SA, Ramsay JD, Kappmeyer LS, Lau AO, Ojo KK, Van Voorhis WC, et al. Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasite Vectors. 2015; 8: 33 10.1186/s13071-014-0611-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

