The Mechanics of Mitotic Cell Rounding
- PMID: 32850812
- PMCID: PMC7423972
- DOI: 10.3389/fcell.2020.00687
The Mechanics of Mitotic Cell Rounding
Abstract
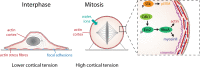
When animal cells enter mitosis, they round up to become spherical. This shape change is accompanied by changes in mechanical properties. Multiple studies using different measurement methods have revealed that cell surface tension, intracellular pressure and cortical stiffness increase upon entry into mitosis. These cell-scale, biophysical changes are driven by alterations in the composition and architecture of the contractile acto-myosin cortex together with osmotic swelling and enable a mitotic cell to exert force against the environment. When the ability of cells to round is limited, for example by physical confinement, cells suffer severe defects in spindle assembly and cell division. The requirement to push against the environment to create space for spindle formation is especially important for cells dividing in tissues. Here we summarize the evidence and the tools used to show that cells exert rounding forces in mitosis in vitro and in vivo, review the molecular basis for this force generation and discuss its function for ensuring successful cell division in single cells and for cells dividing in normal or diseased tissues.
Keywords: Ect2; actin cortex; cell mechanics; ezrin; mitosis; mitotic rounding; myosin; osmotic pressure.
Copyright © 2020 Taubenberger, Baum and Matthews.
Figures

Similar articles
-
Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding.Nature. 2011 Jan 13;469(7329):226-30. doi: 10.1038/nature09642. Epub 2011 Jan 2. Nature. 2011. PMID: 21196934
-
Genome-scale single-cell mechanical phenotyping reveals disease-related genes involved in mitotic rounding.Nat Commun. 2017 Nov 2;8(1):1266. doi: 10.1038/s41467-017-01147-6. Nat Commun. 2017. PMID: 29097687 Free PMC article.
-
F-Actin Interactome Reveals Vimentin as a Key Regulator of Actin Organization and Cell Mechanics in Mitosis.Dev Cell. 2020 Jan 27;52(2):210-222.e7. doi: 10.1016/j.devcel.2019.12.011. Epub 2020 Jan 9. Dev Cell. 2020. PMID: 31928973 Free PMC article.
-
Coupling changes in cell shape to chromosome segregation.Nat Rev Mol Cell Biol. 2016 Aug;17(8):511-21. doi: 10.1038/nrm.2016.75. Epub 2016 Jun 29. Nat Rev Mol Cell Biol. 2016. PMID: 27353479 Review.
-
Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis.Semin Cell Dev Biol. 2014 Oct;34:109-15. doi: 10.1016/j.semcdb.2014.02.015. Epub 2014 Mar 4. Semin Cell Dev Biol. 2014. PMID: 24607328 Review.
Cited by
-
Cell Death by Entosis: Triggers, Molecular Mechanisms and Clinical Significance.Int J Mol Sci. 2022 Apr 30;23(9):4985. doi: 10.3390/ijms23094985. Int J Mol Sci. 2022. PMID: 35563375 Free PMC article. Review.
-
Oncogenic Ras deregulates cell-substrate interactions during mitotic rounding and respreading to alter cell division orientation.Curr Biol. 2023 Jul 10;33(13):2728-2741.e3. doi: 10.1016/j.cub.2023.05.061. Epub 2023 Jun 20. Curr Biol. 2023. PMID: 37343559 Free PMC article.
-
Fused Imidazopyrazine-Based Tubulin Polymerization Inhibitors Inhibit Neuroblastoma Cell Function.ACS Med Chem Lett. 2023 Aug 25;14(9):1284-1294. doi: 10.1021/acsmedchemlett.3c00298. eCollection 2023 Sep 14. ACS Med Chem Lett. 2023. PMID: 37736192 Free PMC article.
-
Deficiency of mastl, a mitotic regulator, results in cell detachment from developing tissues of zebrafish embryos.Front Cell Dev Biol. 2024 Mar 11;12:1375655. doi: 10.3389/fcell.2024.1375655. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38533088 Free PMC article.
-
Contractility-induced self-organization of smooth muscle cells: from multilayer cell sheets to dynamic three-dimensional clusters.Commun Biol. 2023 Mar 11;6(1):262. doi: 10.1038/s42003-023-04578-8. Commun Biol. 2023. PMID: 36906689 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

