MicroRNA-27b-3p Promotes Tumor Progression and Metastasis by Inhibiting Peroxisome Proliferator-Activated Receptor Gamma in Triple-Negative Breast Cancer
- PMID: 32850439
- PMCID: PMC7419677
- DOI: 10.3389/fonc.2020.01371
MicroRNA-27b-3p Promotes Tumor Progression and Metastasis by Inhibiting Peroxisome Proliferator-Activated Receptor Gamma in Triple-Negative Breast Cancer
Abstract
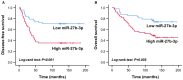
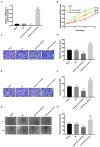
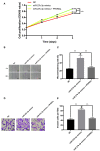
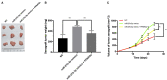
Introduction: The role and underlying mechanisms of miR-27b-3p in triple-negative breast cancer (TNBC) remains unclear. Methods: miR-27b-3p expression level was evaluated in 99 TNBC patients with a median follow-up time of 133 months. The biological functions of miR-27b-3p by targeting PPARG were assessed by luciferase reporter assay, CCK-8 assay, Transwell assay, wound healing assay, western blot analysis and xenograft models. Results: High level of miR-27b-3p expression was found to confer poor prognosis in TNBC patients. MiR-27b-3p overexpression increased TNBC cell proliferation, migration, invasion, and metastasis. Our data suggested peroxisome proliferator-activated receptor gamma (PPARG) was a target of miR-27b-3p. The capacity of miR-27b-3p to induce TNBC progression and metastasis depended on its inhibition of the PPARG expression. Furthermore, restoring PPARG expression reversed the effect of miR-27b-3p overexpression. Mechanistically, miR-27b-3p regulated metastasis-related pathways through PPARG by promoting epithelial-mesenchymal transition. By suppressing PPARG, miR-27b-3p could also activate transcription factors Snail and NF-κB, thereby promoting metastasis. Conclusions: miR-27b-3p promotes TNBC progression and metastasis by inhibiting PPARG. MiR-27b-3p may be a potential prognostic marker of TNBC, and PPARG may be a potential molecular therapeutic target of TNBC.
Keywords: PPARG; metastasis; microRNA-27b-3p; prognosis; triple-negative breast cancer.
Copyright © 2020 Shen, Song, Ren, Xu, Zhou, Liang and Sun.
Figures

Similar articles
-
MiR-25-3p promotes the proliferation of triple negative breast cancer by targeting BTG2.Mol Cancer. 2018 Jan 8;17(1):4. doi: 10.1186/s12943-017-0754-0. Mol Cancer. 2018. PMID: 29310680 Free PMC article.
-
MiR-508-3p inhibits cell invasion and epithelial-mesenchymal transition by targeting ZEB1 in triple-negative breast cancer.Eur Rev Med Pharmacol Sci. 2018 Oct;22(19):6379-6385. doi: 10.26355/eurrev_201810_16050. Eur Rev Med Pharmacol Sci. 2018. PMID: 30338806
-
MicroRNA-590-3p inhibits invasion and metastasis in triple-negative breast cancer by targeting Slug.Am J Cancer Res. 2020 Mar 1;10(3):965-974. eCollection 2020. Am J Cancer Res. 2020. PMID: 32266103 Free PMC article.
-
Tumor tissue microRNA expression in association with triple-negative breast cancer outcomes.Breast Cancer Res Treat. 2015 Jul;152(1):183-191. doi: 10.1007/s10549-015-3460-x. Epub 2015 Jun 11. Breast Cancer Res Treat. 2015. PMID: 26062749 Free PMC article.
-
Lipocalin 2: a potential therapeutic target for breast cancer metastasis.Onco Targets Ther. 2018 Nov 13;11:8099-8106. doi: 10.2147/OTT.S181223. eCollection 2018. Onco Targets Ther. 2018. PMID: 30519052 Free PMC article. Review.
Cited by
-
Ketamine promotes breast tumor growth in a mouse breast tumor model involving with high expression of miR-27b-3p and EGFR.Invest New Drugs. 2022 Dec;40(6):1165-1172. doi: 10.1007/s10637-022-01291-x. Epub 2022 Aug 9. Invest New Drugs. 2022. PMID: 35943683
-
Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer.Front Pharmacol. 2020 Dec 9;11:582719. doi: 10.3389/fphar.2020.582719. eCollection 2020. Front Pharmacol. 2020. PMID: 33536908 Free PMC article.
-
miRNAs as short non-coding RNAs in regulating doxorubicin resistance.J Cell Commun Signal. 2023 Dec;17(4):1181-1202. doi: 10.1007/s12079-023-00789-0. Epub 2023 Nov 29. J Cell Commun Signal. 2023. PMID: 38019354 Free PMC article. Review.
-
Targeting drug resistance in breast cancer: the potential of miRNA and nanotechnology-driven delivery systems.Nanoscale Adv. 2024 Nov 12;6(24):6079-6095. doi: 10.1039/d4na00660g. eCollection 2024 Dec 3. Nanoscale Adv. 2024. PMID: 39569336 Free PMC article. Review.
-
Research progress of nanocarriers for gene therapy targeting abnormal glucose and lipid metabolism in tumors.Drug Deliv. 2021 Dec;28(1):2329-2347. doi: 10.1080/10717544.2021.1995081. Drug Deliv. 2021. PMID: 34730054 Free PMC article. Review.
References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. (2019) 9:176–98. 10.1158/2159-8290.CD-18-1177 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

