Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond
- PMID: 32849586
- PMCID: PMC7411130
- DOI: 10.3389/fimmu.2020.01681
Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond
Abstract
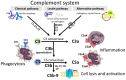
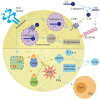
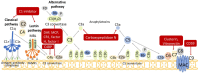
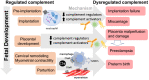
The complement cascade was identified over 100 years ago, yet investigation of its role in pregnancy remains an area of intense research. Complement inhibitors at the maternal-fetal interface prevent inappropriate complement activation to protect the fetus. However, this versatile proteolytic cascade also favorably influences numerous stages of pregnancy, including implantation, fetal development, and labor. Inappropriate complement activation in pregnancy can have adverse lifelong sequelae for both mother and child. This review summarizes the current understanding of complement activation during all stages of pregnancy. In addition, consequences of complement dysregulation during adverse pregnancy outcomes from miscarriage, preeclampsia, and pre-term birth are examined. Finally, future research directions into complement activation during pregnancy are considered.
Keywords: complement; fetal development; innate immunity; preeclampsia; pregnancy; pregnancy loss; preterm birth.
Copyright © 2020 Girardi, Lingo, Fleming and Regal.
Figures

Similar articles
-
Complement activation, a threat to pregnancy.Semin Immunopathol. 2018 Jan;40(1):103-111. doi: 10.1007/s00281-017-0645-x. Epub 2017 Sep 12. Semin Immunopathol. 2018. PMID: 28900713 Review.
-
The complement system and adverse pregnancy outcomes.Mol Immunol. 2015 Sep;67(1):56-70. doi: 10.1016/j.molimm.2015.02.030. Epub 2015 Mar 21. Mol Immunol. 2015. PMID: 25802092 Free PMC article. Review.
-
Role of collectins and complement protein C1q in pregnancy and parturition.Immunobiology. 2016 Nov;221(11):1273-88. doi: 10.1016/j.imbio.2016.06.002. Epub 2016 Jun 13. Immunobiology. 2016. PMID: 27349595 Review.
-
[The immune microenvironment in maternal-fetal interface contributes to the parturition and preterm labor].Sheng Li Xue Bao. 2020 Feb 25;72(1):1-10. Sheng Li Xue Bao. 2020. PMID: 32099979 Review. Chinese.
-
Complement activation and pregnancy failure.Clin Rev Allergy Immunol. 2010 Dec;39(3):153-9. doi: 10.1007/s12016-009-8183-5. Clin Rev Allergy Immunol. 2010. PMID: 19936969 Review.
Cited by
-
The effects of maternal anti-alpha-enolase antibody expression on the brain development in offspring.Clin Exp Immunol. 2022 Dec 15;210(2):187-198. doi: 10.1093/cei/uxac086. Clin Exp Immunol. 2022. PMID: 36149061 Free PMC article.
-
Proteome-defined changes in cellular pathways for decidua and trophoblast tissues associated with location and viability of early-stage pregnancy.Reprod Biol Endocrinol. 2022 Feb 21;20(1):36. doi: 10.1186/s12958-022-00908-3. Reprod Biol Endocrinol. 2022. PMID: 35189928 Free PMC article.
-
Elevated Adipsin and Reduced C5a Levels in the Maternal Serum and Follicular Fluid During Implantation Are Associated With Successful Pregnancy in Obese Women.Front Endocrinol (Lausanne). 2022 Jul 13;13:918320. doi: 10.3389/fendo.2022.918320. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35909516 Free PMC article.
-
Pregnancy in Complement-Mediated Thrombotic Microangiopathy: Maternal and Neonatal Outcomes.Kidney Med. 2023 May 16;5(7):100669. doi: 10.1016/j.xkme.2023.100669. eCollection 2023 Jul. Kidney Med. 2023. PMID: 37492116 Free PMC article.
-
Function of immune cells and effector molecules of the innate immune system in the establishment and maintenance of pregnancy in mammals - A review.Anim Biosci. 2024 Nov;37(11):1821-1833. doi: 10.5713/ab.24.0257. Epub 2024 Aug 23. Anim Biosci. 2024. PMID: 39210819 Free PMC article.
References
-
- Rose MR, Bradley TJ. Evolutionary physiology of the cost of reproduction. Oikos. (1998) 83:443–51. 10.2307/3546672 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

