FgVps9, a Rab5 GEF, Is Critical for DON Biosynthesis and Pathogenicity in Fusarium graminearum
- PMID: 32849361
- PMCID: PMC7418515
- DOI: 10.3389/fmicb.2020.01714
FgVps9, a Rab5 GEF, Is Critical for DON Biosynthesis and Pathogenicity in Fusarium graminearum
Abstract
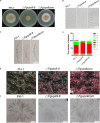
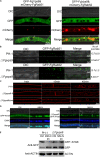
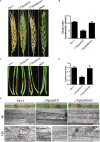
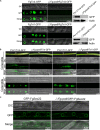
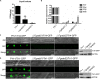
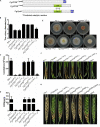
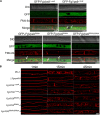
Rab GTPases play an important role in vesicle-mediated membrane trafficking in eukaryotes. Previous studies have demonstrated that deletion of RAB5/VPS21 reduces endocytosis and virulence of fungal phytopathogens in their host plants. However, Rab5 GTPase cycle regulators have not been characterized in Fusarium graminearum, the causal agent of Fusarium head blight (FHB) or head scab disease in cereal crops. In this study, we have identified and characterized a Rab5 guanine nucleotide exchange factor (GEF), the Vps9 homolog FgVps9, in F. graminearum. Yeast two hybrid (Y2H) assays have shown that FgVps9 specifically interacts with the guanosine diphosphate (GDP)-bound (inactive) forms of FgRab51 and FgRab52, the Rab5 isoforms in F. graminearum. Deletion of FgVPS9 shows impaired fungal growth and conidiation. Pathogenicity assays indicate that deletion of FgVPS9 can significantly decrease the virulence of F. graminearum in wheat. Cytological analyses have indicated that FgVps9 colocalizes with FgRab51 and FgRab52 on early endosomes and regulates endocytosis and autophagy processes. Gene expression and cytological examination have shown that FgVps9 and FgRab51 or FgRab52 function in concert to control deoxynivalenol (DON) biosynthesis by regulating the expression of trichothecene biosynthesis-related genes and toxisome biogenesis. Taken together, FgVps9 functions as a GEF for FgRab51 and FgRab52 to regulate endocytosis, which, as a basic cellular function, has significant impact on the vegetative growth, asexual development, autophagy, DON production, and plant infection in F. graminearum.
Keywords: DON; FgVps9; Fusarium graminearum; endocytosis; guanine nucleotide exchange factor; pathogenicity.
Copyright © 2020 Yang, Li, Chen, Zhang, Liao, Yun, Zheng, Abubakar, Li, Wang and Zhou.
Figures

Similar articles
-
FgVAC1 is an Essential Gene Required for Golgi-to-Vacuole Transport and Fungal Development in Fusarium graminearum.J Microbiol. 2024 Aug;62(8):649-660. doi: 10.1007/s12275-024-00160-x. Epub 2024 Jul 30. J Microbiol. 2024. PMID: 39080148 Free PMC article.
-
The Dynamin-Like GTPase FgSey1 Plays a Critical Role in Fungal Development and Virulence in Fusarium graminearum.Appl Environ Microbiol. 2020 May 19;86(11):e02720-19. doi: 10.1128/AEM.02720-19. Print 2020 May 19. Appl Environ Microbiol. 2020. PMID: 32220839 Free PMC article.
-
Nucleoside Diphosphate Kinase FgNdpk Is Required for DON Production and Pathogenicity by Regulating the Growth and Toxisome Formation of Fusarium graminearum.J Agric Food Chem. 2024 May 1;72(17):9637-9646. doi: 10.1021/acs.jafc.4c00593. Epub 2024 Apr 20. J Agric Food Chem. 2024. PMID: 38642053
-
Transcriptomics of cereal-Fusarium graminearum interactions: what we have learned so far.Mol Plant Pathol. 2018 Mar;19(3):764-778. doi: 10.1111/mpp.12561. Epub 2017 Jun 7. Mol Plant Pathol. 2018. PMID: 28411402 Free PMC article. Review.
-
Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management.Annu Rev Phytopathol. 2019 Aug 25;57:15-39. doi: 10.1146/annurev-phyto-082718-100318. Epub 2019 Mar 20. Annu Rev Phytopathol. 2019. PMID: 30893009 Review.
Cited by
-
Alleviating vacuolar transport improves cellulase production in Trichoderma reesei.Appl Microbiol Biotechnol. 2023 Apr;107(7-8):2483-2499. doi: 10.1007/s00253-023-12478-4. Epub 2023 Mar 14. Appl Microbiol Biotechnol. 2023. PMID: 36917273
-
FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum.J Fungi (Basel). 2023 Jan 21;9(2):145. doi: 10.3390/jof9020145. J Fungi (Basel). 2023. PMID: 36836259 Free PMC article.
-
The GTPase-Activating Protein FgGyp1 Is Important for Vegetative Growth, Conidiation, and Virulence and Negatively Regulates DON Biosynthesis in Fusarium graminearium.Front Microbiol. 2021 Jan 21;12:621519. doi: 10.3389/fmicb.2021.621519. eCollection 2021. Front Microbiol. 2021. PMID: 33552040 Free PMC article.
-
The Small GTPase FgRab1 Plays Indispensable Roles in the Vegetative Growth, Vesicle Fusion, Autophagy and Pathogenicity of Fusarium graminearum.Int J Mol Sci. 2022 Jan 14;23(2):895. doi: 10.3390/ijms23020895. Int J Mol Sci. 2022. PMID: 35055095 Free PMC article.
-
FgVAC1 is an Essential Gene Required for Golgi-to-Vacuole Transport and Fungal Development in Fusarium graminearum.J Microbiol. 2024 Aug;62(8):649-660. doi: 10.1007/s12275-024-00160-x. Epub 2024 Jul 30. J Microbiol. 2024. PMID: 39080148 Free PMC article.
References
-
- Alexander N. J., Proctor R. H., McCormick S. P. (2009). Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 28 198–215. 10.1080/15569540903092142 - DOI
-
- Bai G., Shaner G. (1994). Scab of wheat: prospects for control. Plant Dis. 78 760–766. 10.1094/PD-78-0760 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

