Histidine-Rich Defensins from the Solanaceae and Brasicaceae Are Antifungal and Metal Binding Proteins
- PMID: 32847065
- PMCID: PMC7557933
- DOI: 10.3390/jof6030145
Histidine-Rich Defensins from the Solanaceae and Brasicaceae Are Antifungal and Metal Binding Proteins
Abstract
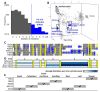
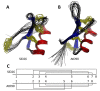
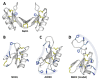
Plant defensins are best known for their antifungal activity and contribution to the plant immune system. The defining feature of plant defensins is their three-dimensional structure known as the cysteine stabilized alpha-beta motif. This protein fold is remarkably tolerant to sequence variation with only the eight cysteines that contribute to the stabilizing disulfide bonds absolutely conserved across the family. Mature defensins are typically 46-50 amino acids in length and are enriched in lysine and/or arginine residues. Examination of a database of approximately 1200 defensin sequences revealed a subset of defensin sequences that were extended in length and were enriched in histidine residues leading to their classification as histidine-rich defensins (HRDs). Using these initial HRD sequences as a query, a search of the available sequence databases identified over 750 HRDs in solanaceous plants and 20 in brassicas. Histidine residues are known to contribute to metal binding functions in proteins leading to the hypothesis that HRDs would have metal binding properties. A selection of the HRD sequences were recombinantly expressed and purified and their antifungal and metal binding activity was characterized. Of the four HRDs that were successfully expressed all displayed some level of metal binding and two of four had antifungal activity. Structural characterization of the other HRDs identified a novel pattern of disulfide linkages in one of the HRDs that is predicted to also occur in HRDs with similar cysteine spacing. Metal binding by HRDs represents a specialization of the plant defensin fold outside of antifungal activity.
Keywords: antifungal; histidine; metal binding; plant defensin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
NMR structure and conformational dynamics of AtPDFL2.1, a defensin-like peptide from Arabidopsis thaliana.Biochim Biophys Acta. 2016 Dec;1864(12):1739-1747. doi: 10.1016/j.bbapap.2016.08.017. Epub 2016 Aug 31. Biochim Biophys Acta. 2016. PMID: 27592418
-
Structure of Petunia hybrida defensin 1, a novel plant defensin with five disulfide bonds.Biochemistry. 2003 Jul 15;42(27):8214-22. doi: 10.1021/bi034379o. Biochemistry. 2003. PMID: 12846570
-
The evolution, function and mechanisms of action for plant defensins.Semin Cell Dev Biol. 2019 Apr;88:107-118. doi: 10.1016/j.semcdb.2018.02.004. Epub 2018 Feb 23. Semin Cell Dev Biol. 2019. PMID: 29432955 Review.
-
The nuclear magnetic resonance solution structure of the synthetic AhPDF1.1b plant defensin evidences the structural feature within the γ-motif.Biochemistry. 2014 Dec 16;53(49):7745-54. doi: 10.1021/bi501285k. Epub 2014 Dec 5. Biochemistry. 2014. PMID: 25419866
-
Plant defensins.Planta. 2002 Dec;216(2):193-202. doi: 10.1007/s00425-002-0902-6. Epub 2002 Oct 8. Planta. 2002. PMID: 12447532 Review.
Cited by
-
Proteomic response of Escherichia coli to a membrane lytic and iron chelating truncated Amaranthus tricolor defensin.BMC Microbiol. 2021 Apr 12;21(1):110. doi: 10.1186/s12866-021-02176-4. BMC Microbiol. 2021. PMID: 33845758 Free PMC article.
-
New Insights into Involvement of Low Molecular Weight Proteins in Complex Defense Mechanisms in Higher Plants.Int J Mol Sci. 2024 Aug 5;25(15):8531. doi: 10.3390/ijms25158531. Int J Mol Sci. 2024. PMID: 39126099 Free PMC article. Review.
-
Antifungal Peptides.J Fungi (Basel). 2021 May 31;7(6):437. doi: 10.3390/jof7060437. J Fungi (Basel). 2021. PMID: 34072631 Free PMC article.
-
The structural repertoire of Fusarium oxysporum f. sp. lycopersici effectors revealed by experimental and computational studies.Elife. 2024 Feb 27;12:RP89280. doi: 10.7554/eLife.89280. Elife. 2024. PMID: 38411527 Free PMC article.
References
-
- Van der Weerden N.L., Anderson M.A. Plant Defensins: Common Fold, Multiple Functions. Fungal Biol. Rev. 2013;26:121–131. doi: 10.1016/j.fbr.2012.08.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

