Hif-1α Inhibitors Could Successfully Inhibit the Progression of Differentiated Thyroid Cancer in Vitro
- PMID: 32847004
- PMCID: PMC7558478
- DOI: 10.3390/ph13090208
Hif-1α Inhibitors Could Successfully Inhibit the Progression of Differentiated Thyroid Cancer in Vitro
Abstract
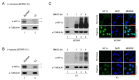
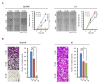
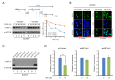
Hypoxia-inducible factor (HIF)-1α plays an important role in cancer progression. In various cancers, including thyroid cancer, overexpression of HIF-1α is related to poor prognosis or treatment response. However, few studies have investigated the role of HIF-1α inhibition in thyroid cancer progression. We evaluated the utility of the HIF-1α inhibitor IDF-11774 in vitro utilizing two thyroid cancer cell lines, K1 and BCPAP. Both cell lines were tested to elucidate the effects of IDF-11774 on cell proliferation and migration using soft agar and invasion assays. Here, we found that a reduction of HIF-1α expression in BCPAP cells was observed after treatment with IDF-11774 in a dose-dependent manner. Moreover, cell proliferation, migration, and anchorage-independent growth were effectively inhibited by IDF-11774 in BCPAP cells but not in K1 cells. Additionally, invasion of BCPAP but not K1 cells was controlled with IDF-11774 in a dose-dependent manner. Our findings suggest that promoting the degradation of HIF-1α could be a strategy to manage progression and that HIF-1α inhibitors are potent drugs for thyroid cancer treatment.
Keywords: HIF-1α; IDF-11774; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
IDF-11774 Induces Cell Cycle Arrest and Apoptosis by Inhibiting HIF-1α in Gastric Cancer.Pharmaceutics. 2023 Dec 13;15(12):2772. doi: 10.3390/pharmaceutics15122772. Pharmaceutics. 2023. PMID: 38140111 Free PMC article.
-
Anti-Tumor Effect of IDF-11774, an Inhibitor of Hypoxia-Inducible Factor-1, on Melanoma.Biomol Ther (Seoul). 2022 Sep 1;30(5):465-472. doi: 10.4062/biomolther.2022.061. Epub 2022 Jun 17. Biomol Ther (Seoul). 2022. PMID: 35712870 Free PMC article.
-
The novel hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth.Cell Death Dis. 2017 Jun 1;8(6):e2843. doi: 10.1038/cddis.2017.235. Cell Death Dis. 2017. PMID: 28569777 Free PMC article.
-
Bridging hypoxia, inflammation and estrogen receptors in thyroid cancer progression.Biomed Pharmacother. 2014 Feb;68(1):1-5. doi: 10.1016/j.biopha.2013.10.013. Epub 2013 Nov 15. Biomed Pharmacother. 2014. PMID: 24286852 Review.
-
Is the hypoxia-inducible factor pathway important in gastric cancer?Eur J Cancer. 2005 Dec;41(18):2792-805. doi: 10.1016/j.ejca.2005.09.008. Epub 2005 Nov 14. Eur J Cancer. 2005. PMID: 16290133 Review.
Cited by
-
HIF-1α signaling: Essential roles in tumorigenesis and implications in targeted therapies.Genes Dis. 2023 Mar 30;11(1):234-251. doi: 10.1016/j.gendis.2023.02.039. eCollection 2024 Jan. Genes Dis. 2023. PMID: 37588219 Free PMC article. Review.
-
Advances in the molecular mechanism and targeted therapy of radioactive-iodine refractory differentiated thyroid cancer.Med Oncol. 2023 Jul 31;40(9):258. doi: 10.1007/s12032-023-02098-3. Med Oncol. 2023. PMID: 37524925 Review.
-
Small molecule inhibitors targeting the cancers.MedComm (2020). 2022 Oct 13;3(4):e181. doi: 10.1002/mco2.181. eCollection 2022 Dec. MedComm (2020). 2022. PMID: 36254250 Free PMC article. Review.
-
Poorly Differentiated Thyroid Carcinoma: Single Centre Experience and Review of the Literature.J Clin Med. 2021 Nov 12;10(22):5258. doi: 10.3390/jcm10225258. J Clin Med. 2021. PMID: 34830540 Free PMC article. Review.
-
In Vitro Investigation of HIF-1α as a Therapeutic Target for Thyroid-Associated Ophthalmopathy.Endocrinol Metab (Seoul). 2024 Oct;39(5):767-776. doi: 10.3803/EnM.2024.1952. Epub 2024 Oct 16. Endocrinol Metab (Seoul). 2024. PMID: 39410849 Free PMC article.
References
-
- Durante C., Haddy N., Baudin E., Leboulleux S., Hartl D., Travagli J.P., Caillou B., Ricard M., Lumbroso J.D., de Vathaire F., et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

