Influenza-Induced Oxidative Stress Sensitizes Lung Cells to Bacterial-Toxin-Mediated Necroptosis
- PMID: 32846120
- PMCID: PMC7570217
- DOI: 10.1016/j.celrep.2020.108062
Influenza-Induced Oxidative Stress Sensitizes Lung Cells to Bacterial-Toxin-Mediated Necroptosis
Abstract
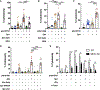
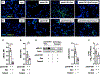
Pneumonias caused by influenza A virus (IAV) co- and secondary bacterial infections are characterized by their severity and high mortality rate. Previously, we have shown that bacterial pore-forming toxin (PFT)-mediated necroptosis is a key driver of acute lung injury during bacterial pneumonia. Here, we evaluate the impact of IAV on PFT-induced acute lung injury during co- and secondary Streptococcus pneumoniae (Spn) infection. We observe that IAV synergistically sensitizes lung epithelial cells for PFT-mediated necroptosis in vitro and in murine models of Spn co-infection and secondary infection. Pharmacoelogical induction of oxidative stress without virus sensitizes cells for PFT-mediated necroptosis. Antioxidant treatment or inhibition of necroptosis reduces disease severity during secondary bacterial infection. Our results advance our understanding on the molecular basis of co- and secondary bacterial infection to influenza and identify necroptosis inhibition and antioxidant therapy as potential intervention strategies.
Keywords: Streptococcus pneumoniae; cell death; co-infections; epithelial cells; inflammation; influenza A virus; necroptosis; oxidative stress; pneumonia; secondary bacterial infection.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

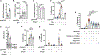


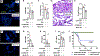
Similar articles
-
GP96 Drives Exacerbation of Secondary Bacterial Pneumonia following Influenza A Virus Infection.mBio. 2021 Jun 29;12(3):e0326920. doi: 10.1128/mBio.03269-20. Epub 2021 Jun 1. mBio. 2021. PMID: 34061598 Free PMC article.
-
Streptococcus pneumoniae binds to host GAPDH on dying lung epithelial cells worsening secondary infection following influenza.Cell Rep. 2021 Jun 15;35(11):109267. doi: 10.1016/j.celrep.2021.109267. Cell Rep. 2021. PMID: 34133917 Free PMC article.
-
Necroptotic Cell Death Promotes Adaptive Immunity Against Colonizing Pneumococci.Front Immunol. 2019 Apr 4;10:615. doi: 10.3389/fimmu.2019.00615. eCollection 2019. Front Immunol. 2019. PMID: 31019504 Free PMC article.
-
Secondary streptococcal infection following influenza.Microbiol Immunol. 2022 Jun;66(6):253-263. doi: 10.1111/1348-0421.12965. Epub 2022 May 26. Microbiol Immunol. 2022. PMID: 35088451 Review.
-
Participation of Necroptosis in the Host Response to Acute Bacterial Pneumonia.J Innate Immun. 2017;9(3):262-270. doi: 10.1159/000455100. Epub 2017 Jan 27. J Innate Immun. 2017. PMID: 28125817 Free PMC article. Review.
Cited by
-
Virus-Induced Changes of the Respiratory Tract Environment Promote Secondary Infections With Streptococcus pneumoniae.Front Cell Infect Microbiol. 2021 Mar 22;11:643326. doi: 10.3389/fcimb.2021.643326. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33828999 Free PMC article. Review.
-
Pandemic Influenza Infection Promotes Streptococcus pneumoniae Infiltration, Necrotic Damage, and Proteomic Remodeling in the Heart.mBio. 2022 Feb 22;13(1):e0325721. doi: 10.1128/mbio.03257-21. Epub 2022 Jan 4. mBio. 2022. PMID: 35089061 Free PMC article.
-
Effects of Contagious Respiratory Pathogens on Breath Biomarkers.Antioxidants (Basel). 2024 Jan 29;13(2):172. doi: 10.3390/antiox13020172. Antioxidants (Basel). 2024. PMID: 38397770 Free PMC article.
-
Novel neuroendocrine role of γ-aminobutyric acid and gastrin-releasing peptide in the host response to influenza infection.Mucosal Immunol. 2023 Jun;16(3):302-311. doi: 10.1016/j.mucimm.2023.03.004. Epub 2023 Mar 24. Mucosal Immunol. 2023. PMID: 36965691 Free PMC article.
-
M1 Macrophages Are More Susceptible to Necroptosis.J Cell Immunol. 2021;3(2):97-102. doi: 10.33696/immunology.3.084. J Cell Immunol. 2021. PMID: 33959729 Free PMC article.
References
-
- Byrn RA, Jones SM, Bennett HB, Bral C, Clark MP, Jacobs MD, Kwong AD, Ledeboer MW, Leeman JR, McNeil CF, et al. (2015). Pre-clinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob. Agents Chemother 59, 1569–1582. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

