IL-32γ potentiates tumor immunity in melanoma
- PMID: 32841222
- PMCID: PMC7526542
- DOI: 10.1172/jci.insight.138772
IL-32γ potentiates tumor immunity in melanoma
Abstract
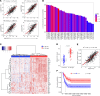
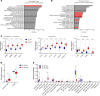
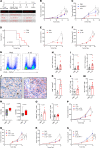
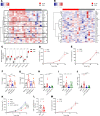
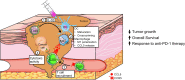
Myeloid cells orchestrate the antitumor immune response and influence the efficacy of immune checkpoint blockade (ICB) therapies. We and others have previously shown that IL-32 mediates DC differentiation and macrophage activation. Here, we demonstrate that IL-32 expression in human melanoma positively correlates with overall survival, response to ICB, and an immune-inflamed tumor microenvironment (TME) enriched in mature DC, M1 macrophages, and CD8+ T cells. Treatment of B16F10 murine melanomas with IL-32 increased the frequencies of activated, tumor-specific CD8+ T cells, leading to the induction of systemic tumor immunity. Our mechanistic in vivo studies revealed a potentially novel role of IL-32 in activating intratumoral DC and macrophages to act in concert to prime CD8+ T cells and recruit them into the TME through CCL5. Thereby, IL-32 treatment reduced tumor growth and rendered ICB-resistant B16F10 tumors responsive to anti-PD-1 therapy without toxicity. Furthermore, increased baseline IL-32 gene expression was associated with response to nivolumab and pembrolizumab in 2 independent cohorts of patients with melanoma, implying that IL-32 is a predictive biomarker for anti-PD-1 therapy. Collectively, this study suggests IL-32 as a potent adjuvant in immunotherapy to enhance the efficacy of ICB in patients with non-T cell-inflamed TME.
Keywords: Cancer immunotherapy; Cellular immune response; Immunology; Melanoma; Oncology.
Conflict of interest statement
Figures

Similar articles
-
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.J Hematol Oncol. 2019 Nov 27;12(1):124. doi: 10.1186/s13045-019-0822-6. J Hematol Oncol. 2019. PMID: 31771616 Free PMC article.
-
Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies.J Immunother Cancer. 2019 Sep 11;7(1):244. doi: 10.1186/s40425-019-0724-8. J Immunother Cancer. 2019. PMID: 31511088 Free PMC article.
-
Targeting the vascular endothelial growth factor receptor-1 by the monoclonal antibody D16F7 to increase the activity of immune checkpoint inhibitors against cutaneous melanoma.Pharmacol Res. 2020 Sep;159:104957. doi: 10.1016/j.phrs.2020.104957. Epub 2020 May 30. Pharmacol Res. 2020. PMID: 32485280
-
The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies.Front Immunol. 2020 May 7;11:784. doi: 10.3389/fimmu.2020.00784. eCollection 2020. Front Immunol. 2020. PMID: 32457745 Free PMC article. Review.
-
Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy.Front Immunol. 2018 Dec 18;9:2905. doi: 10.3389/fimmu.2018.02905. eCollection 2018. Front Immunol. 2018. PMID: 30619269 Free PMC article. Review.
Cited by
-
Interleukin 32 Promotes Foxp3+ Treg Cell Development and CD8+ T Cell Function in Human Esophageal Squamous Cell Carcinoma Microenvironment.Front Cell Dev Biol. 2021 Aug 3;9:704853. doi: 10.3389/fcell.2021.704853. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34414188 Free PMC article.
-
The epiphany derived from T-cell-inflamed profiles: Pan-cancer characterization of CD8A as a biomarker spanning clinical relevance, cancer prognosis, immunosuppressive environment, and treatment responses.Front Genet. 2022 Aug 11;13:974416. doi: 10.3389/fgene.2022.974416. eCollection 2022. Front Genet. 2022. PMID: 36035168 Free PMC article.
-
A Paradoxical Effect of Interleukin-32 Isoforms on Cancer.Front Immunol. 2022 Feb 25;13:837590. doi: 10.3389/fimmu.2022.837590. eCollection 2022. Front Immunol. 2022. PMID: 35281008 Free PMC article. Review.
-
DNA Methylation Modification Map to Predict Tumor Molecular Subtypes and Efficacy of Immunotherapy in Bladder Cancer.Front Cell Dev Biol. 2021 Dec 3;9:760369. doi: 10.3389/fcell.2021.760369. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34926451 Free PMC article.
-
Cannabinoid Receptor Type-2 in B Cells Is Associated with Tumor Immunity in Melanoma.Cancers (Basel). 2021 Apr 16;13(8):1934. doi: 10.3390/cancers13081934. Cancers (Basel). 2021. PMID: 33923757 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

