Cardiomyocyte adhesion and hyperadhesion differentially require ERK1/2 and plakoglobin
- PMID: 32841221
- PMCID: PMC7526536
- DOI: 10.1172/jci.insight.140066
Cardiomyocyte adhesion and hyperadhesion differentially require ERK1/2 and plakoglobin
Abstract
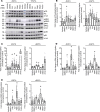
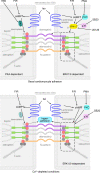
Arrhythmogenic cardiomyopathy (AC) is a heart disease often caused by mutations in genes coding for desmosomal proteins, including desmoglein-2 (DSG2), plakoglobin (PG), and desmoplakin (DP). Therapy is based on symptoms and limiting arrhythmia, because the mechanisms by which desmosomal components control cardiomyocyte function are largely unknown. A new paradigm could be to stabilize desmosomal cardiomyocyte adhesion and hyperadhesion, which renders desmosomal adhesion independent from Ca2+. Here, we further characterized the mechanisms behind enhanced cardiomyocyte adhesion and hyperadhesion. Dissociation assays performed in HL-1 cells and murine ventricular cardiac slice cultures allowed us to define a set of signaling pathways regulating cardiomyocyte adhesion under basal and hyperadhesive conditions. Adrenergic signaling, activation of PKC, and inhibition of p38MAPK enhanced cardiomyocyte adhesion, referred to as positive adhesiotropy, and induced hyperadhesion. Activation of ERK1/2 paralleled positive adhesiotropy, whereas adrenergic signaling induced PG phosphorylation at S665 under both basal and hyperadhesive conditions. Adrenergic signaling and p38MAPK inhibition recruited DSG2 to cell junctions. In PG-deficient mice with an AC phenotype, only PKC activation and p38MAPK inhibition enhanced cardiomyocyte adhesion. Our results demonstrate that cardiomyocyte adhesion can be stabilized by different signaling mechanisms, which are in part offset in PG-deficient AC.
Keywords: Arrhythmias; Cardiology; Cardiovascular disease; Cell migration/adhesion.
Conflict of interest statement
Figures




Similar articles
-
Cardiomyocyte cohesion is increased after ADAM17 inhibition.Front Cell Dev Biol. 2023 Jan 17;11:1021595. doi: 10.3389/fcell.2023.1021595. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36733457 Free PMC article.
-
Cholinergic signaling impairs cardiomyocyte cohesion.Acta Physiol (Oxf). 2022 Nov;236(3):e13881. doi: 10.1111/apha.13881. Epub 2022 Sep 16. Acta Physiol (Oxf). 2022. PMID: 36039679
-
Adrenergic Signaling Strengthens Cardiac Myocyte Cohesion.Circ Res. 2017 Apr 14;120(8):1305-1317. doi: 10.1161/CIRCRESAHA.116.309631. Epub 2017 Mar 13. Circ Res. 2017. PMID: 28289018
-
New insights into desmosome regulation and pemphigus blistering as a desmosome-remodeling disease.Kaohsiung J Med Sci. 2013 Jan;29(1):1-13. doi: 10.1016/j.kjms.2012.08.001. Epub 2012 Oct 12. Kaohsiung J Med Sci. 2013. PMID: 23257250 Review.
-
Beyond cell-cell adhesion: Plakoglobin and the regulation of tumorigenesis and metastasis.Oncotarget. 2017 May 9;8(19):32270-32291. doi: 10.18632/oncotarget.15650. Oncotarget. 2017. PMID: 28416759 Free PMC article. Review.
Cited by
-
In Vivo Approaches to Understand Arrhythmogenic Cardiomyopathy: Perspectives on Animal Models.Cells. 2024 Jul 27;13(15):1264. doi: 10.3390/cells13151264. Cells. 2024. PMID: 39120296 Free PMC article. Review.
-
Catalytic antibodies in arrhythmogenic cardiomyopathy patients cleave desmoglein 2 and N-cadherin and impair cardiomyocyte cohesion.Cell Mol Life Sci. 2023 Jul 14;80(8):203. doi: 10.1007/s00018-023-04853-1. Cell Mol Life Sci. 2023. PMID: 37450050 Free PMC article.
-
EGFR inhibition leads to enhanced desmosome assembly and cardiomyocyte cohesion via ROCK activation.JCI Insight. 2023 Mar 22;8(6):e163763. doi: 10.1172/jci.insight.163763. JCI Insight. 2023. PMID: 36795511 Free PMC article.
-
Cardiomyocyte cohesion is increased after ADAM17 inhibition.Front Cell Dev Biol. 2023 Jan 17;11:1021595. doi: 10.3389/fcell.2023.1021595. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36733457 Free PMC article.
-
Electrophysiological Remodeling: Cardiac T-Tubules and ß-Adrenoceptors.Cells. 2021 Sep 17;10(9):2456. doi: 10.3390/cells10092456. Cells. 2021. PMID: 34572106 Free PMC article. Review.
References
-
- Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol. 2006;85(2):69–82. doi: 10.1016/j.ejcb.2005.11.003. - DOI - PubMed
-
- Al-Amoudi A, Frangakis AS. Structural studies on desmosomes. Biochem Soc Trans. 2008;36(Pt 2):181–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

