Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway
- PMID: 32839503
- PMCID: PMC8149448
- DOI: 10.1038/s41401-020-0495-2
Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway
Abstract
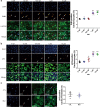
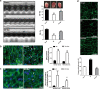
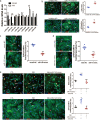
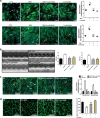
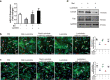
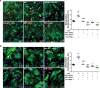
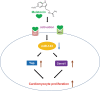
The neonatal heart possesses the ability to proliferate and the capacity to regenerate after injury; however, the mechanisms underlying these processes are not fully understood. Melatonin has been shown to protect the heart against myocardial injury through mitigating oxidative stress, reducing apoptosis, inhibiting mitochondrial fission, etc. In this study, we investigated whether melatonin regulated cardiomyocyte proliferation and promoted cardiac repair in mice with myocardial infarction (MI), which was induced by ligation of the left anterior descending coronary artery. We showed that melatonin administration significantly improved the cardiac functions accompanied by markedly enhanced cardiomyocyte proliferation in MI mice. In neonatal mouse cardiomyocytes, treatment with melatonin (1 μM) greatly suppressed miR-143-3p levels. Silencing of miR-143-3p stimulated cardiomyocytes to re-enter the cell cycle. On the contrary, overexpression of miR-143-3p inhibited the mitosis of cardiomyocytes and abrogated cardiomyocyte mitosis induced by exposure to melatonin. Moreover, Yap and Ctnnd1 were identified as the target genes of miR-143-3p. In cardiomyocytes, inhibition of miR-143-3p increased the protein expression of Yap and Ctnnd1. Melatonin treatment also enhanced Yap and Ctnnd1 protein levels. Furthermore, Yap siRNA and Ctnnd1 siRNA attenuated melatonin-induced cell cycle re-entry of cardiomyocytes. We showed that the effect of melatonin on cardiomyocyte proliferation and cardiac regeneration was impeded by the melatonin receptor inhibitor luzindole. Silencing miR-143-3p abrogated the inhibition of luzindole on cardiomyocyte proliferation. In addition, both MT1 and MT2 siRNA could cancel the beneficial effects of melatonin on cardiomyocyte proliferation. Collectively, the results suggest that melatonin induces cardiomyocyte proliferation and heart regeneration after MI by regulating the miR-143-3p/Yap/Ctnnd1 signaling pathway, providing a new therapeutic strategy for cardiac regeneration.
Keywords: Ctnnd1; Yap; cardiac repair; cardiomyocyte proliferation; luzindole; melatonin; miR-143-3p; myocardial infarction.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Cannabidiol represses miR-143 to promote cardiomyocyte proliferation and heart regeneration after myocardial infarction.Eur J Pharmacol. 2024 Jan 15;963:176245. doi: 10.1016/j.ejphar.2023.176245. Epub 2023 Dec 3. Eur J Pharmacol. 2024. PMID: 38052413
-
Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity.Circ Res. 2015 Jan 2;116(1):70-9. doi: 10.1161/CIRCRESAHA.116.304472. Epub 2014 Oct 10. Circ Res. 2015. PMID: 25305307 Free PMC article.
-
Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction.Clin Sci (Lond). 2017 Dec 8;131(24):2919-2932. doi: 10.1042/CS20171256. Print 2017 Dec 15. Clin Sci (Lond). 2017. PMID: 29162747
-
Non-coding RNA therapeutics for cardiac regeneration.Cardiovasc Res. 2021 Feb 22;117(3):674-693. doi: 10.1093/cvr/cvaa071. Cardiovasc Res. 2021. PMID: 32215566 Free PMC article. Review.
-
Understanding cardiomyocyte proliferation: an insight into cell cycle activity.Cell Mol Life Sci. 2017 Mar;74(6):1019-1034. doi: 10.1007/s00018-016-2375-y. Epub 2016 Sep 30. Cell Mol Life Sci. 2017. PMID: 27695872 Free PMC article. Review.
Cited by
-
The Therapeutic Strategies Targeting Mitochondrial Metabolism in Cardiovascular Disease.Pharmaceutics. 2022 Dec 9;14(12):2760. doi: 10.3390/pharmaceutics14122760. Pharmaceutics. 2022. PMID: 36559254 Free PMC article. Review.
-
Melatonin Promotes Antler Growth by Accelerating MT1-Mediated Mesenchymal Cell Differentiation and Inhibiting VEGF-Induced Degeneration of Chondrocytes.Int J Mol Sci. 2022 Jan 11;23(2):759. doi: 10.3390/ijms23020759. Int J Mol Sci. 2022. PMID: 35054949 Free PMC article.
-
Single-Cell RNA-Seq Analysis Reveals Lung Epithelial Cell Type-Specific Responses to HDM and Regulation by Tet1.Genes (Basel). 2022 May 14;13(5):880. doi: 10.3390/genes13050880. Genes (Basel). 2022. PMID: 35627266 Free PMC article.
-
New insights into the role of melatonin in diabetic cardiomyopathy.Pharmacol Res Perspect. 2022 Feb;10(1):e00904. doi: 10.1002/prp2.904. Pharmacol Res Perspect. 2022. PMID: 35005848 Free PMC article. Review.
-
Mechanism of METTL3-Mediated m6A Modification in Cardiomyocyte Pyroptosis and Myocardial Ischemia-Reperfusion Injury.Cardiovasc Drugs Ther. 2023 Jun;37(3):435-448. doi: 10.1007/s10557-021-07300-0. Epub 2022 Jan 23. Cardiovasc Drugs Ther. 2023. PMID: 35066738
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

