TRPA1 as a therapeutic target for nociceptive pain
- PMID: 32838583
- PMCID: PMC7610834
- DOI: 10.1080/14728222.2020.1815191
TRPA1 as a therapeutic target for nociceptive pain
Abstract
Introduction: Chronic pain affects approximatively 30-50% of the population globally. Pathologies such as migraine, diabetic neuropathy, nerve injury and treatment with chemotherapeutic agents, can induce chronic pain. Members of the transient receptor potential (TRP) channels, including the TRP ankyrin 1 (TRPA1), have a major role in pain.
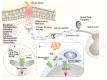
Areas covered: We focus on TRPA1 as a therapeutic target for pain relief. The structure, localization, and activation of the channel and its implication in different pathways to signal pain are described. This paper underlines the role of pharmacological interventions on TRPA1 to reduce pain in numerous pain conditions. We conducted a literature search in PubMed up to and including July 2020.
Expert opinion: Our understanding of the molecular mechanisms underlying the sensitization of central and peripheral nociceptive pathways is limited. Preclinical evidence indicates that, in murine models of pain diseases, numerous mechanisms converge on the pathway that encompasses oxidative stress and Schwann cell TRPA1 to sustain chronic pain. Programs to identify and develop treatments to attenuate TRPA1-mediated chronic pain have emerged from this knowledge. Antagonists explored as a novel class of analgesics have a new and promising target in the TRPA1 expressed by peripheral glial cells.
Keywords: Inflammatory pain; TRP ankyrin 1 (TRPA1); Transient Receptor Potential (TRP) channels; neuropathic pain; nociception.
Conflict of interest statement
F De Logu, P Geppetti and R.Nassini are founding scientists of FloNext Srl. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No potential conflict of interest was reported by the authors.
Figures

Similar articles
-
Is TRPA1 Burning Down TRPV1 as Druggable Target for the Treatment of Chronic Pain?Int J Mol Sci. 2019 Jun 14;20(12):2906. doi: 10.3390/ijms20122906. Int J Mol Sci. 2019. PMID: 31197115 Free PMC article. Review.
-
TRPA1 involvement in analgesia induced by Tabernaemontana catharinensis ethyl acetate fraction in mice.Phytomedicine. 2019 Feb 15;54:248-258. doi: 10.1016/j.phymed.2018.09.201. Epub 2018 Sep 18. Phytomedicine. 2019. PMID: 30668375
-
Analgesic effects of the novel semicarbazide-sensitive amine oxidase inhibitor SZV 1287 in mouse pain models with neuropathic mechanisms: Involvement of transient receptor potential vanilloid 1 and ankyrin 1 receptors.Pharmacol Res. 2018 May;131:231-243. doi: 10.1016/j.phrs.2018.02.006. Epub 2018 Feb 10. Pharmacol Res. 2018. PMID: 29438782
-
The TRPA1 channel in migraine mechanism and treatment.Br J Pharmacol. 2014 May;171(10):2552-67. doi: 10.1111/bph.12512. Br J Pharmacol. 2014. PMID: 24206166 Free PMC article. Review.
-
TRPA1 antagonists as potential analgesic drugs.Pharmacol Ther. 2012 Feb;133(2):189-204. doi: 10.1016/j.pharmthera.2011.10.008. Epub 2011 Nov 15. Pharmacol Ther. 2012. PMID: 22119554 Review.
Cited by
-
A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain.Brain Sci. 2024 Jun 9;14(6):589. doi: 10.3390/brainsci14060589. Brain Sci. 2024. PMID: 38928589 Free PMC article. Review.
-
The transient receptor potential channels in rheumatoid arthritis: Need to pay more attention.Front Immunol. 2023 Mar 1;14:1127277. doi: 10.3389/fimmu.2023.1127277. eCollection 2023. Front Immunol. 2023. PMID: 36926330 Free PMC article. Review.
-
TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches.Biomolecules. 2023 Oct 22;13(10):1557. doi: 10.3390/biom13101557. Biomolecules. 2023. PMID: 37892239 Free PMC article. Review.
-
TRPA1, TRPV1, and Caffeine: Pain and Analgesia.Int J Mol Sci. 2024 Jul 19;25(14):7903. doi: 10.3390/ijms25147903. Int J Mol Sci. 2024. PMID: 39063144 Free PMC article. Review.
-
Dimethyl Fumarate Attenuates Pain Behaviors in Osteoarthritis Rats via Induction of Nrf2-Mediated Mitochondrial Biogenesis.Mol Pain. 2022 Sep 6;18:17448069221124920. doi: 10.1177/17448069221124920. Online ahead of print. Mol Pain. 2022. PMID: 36065971 Free PMC article.
References
-
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458. [•• Comprehensive review on TRP channels.] - PubMed
-
- Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. [•• Comprehensive review on TRP channels.] - PubMed
-
- Logashina YA, Korolkova YV, Kozlov SA, et al. Biochem. Vol. 84. Pleiades Publishing; 2019. TRPA1 channel as a regulator of neurogenic inflammation and pain: structure, function, role in pathophysiology, and therapeutic potential of ligands; pp. 101–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
