TRIM Proteins in Host Defense and Viral Pathogenesis
- PMID: 32837832
- PMCID: PMC7414267
- DOI: 10.1007/s40588-020-00150-8
TRIM Proteins in Host Defense and Viral Pathogenesis
Abstract
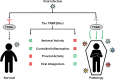
Purpose of review: Tripartite motif (TRIM) proteins are a large group of E3 ubiquitin ligases involved in different cellular functions. Of special interest are their roles in innate immunity, inflammation, and virus replication. We discuss novel roles of TRIM proteins during virus infections that lead to increased pathogenicity.
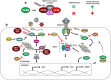
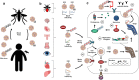
Recent findings: TRIM proteins regulate different antiviral and inflammatory signaling pathways, mostly by promoting ubiquitination of important factors including pattern recognition receptors, adaptor proteins, kinases, and transcription factors that are involved in type I interferon and NF-κB pathways. Therefore, viruses have developed mechanisms to target TRIMs for immune evasion. New evidence is emerging indicating that viruses have the ability to directly use TRIMs and the ubiquitination process to enhance the viral replication cycle and cause increased pathogenesis. A new report on TRIM7 also highlights the potential pro-viral role of TRIMs via ubiquitination of viral proteins and suggests a novel mechanism by which ubiquitination of virus envelope protein may provide determinants of tissue and species tropism.
Summary: TRIM proteins have important functions in promoting host defense against virus infection; however, viruses have adapted to evade TRIM-mediated immune responses and can hijack TRIMs to ultimately increase virus pathogenesis. Only by understanding specific TRIM-virus interactions and by using more in vivo approaches can we learn how to harness TRIM function to develop therapeutic approaches to reduce virus pathogenesis.
Keywords: E3 ubiquitin ligase; Immunity; Pathogenesis; TRIM6; TRIM7; Tripartite motif (TRIM); Type I interferons; Ubiquitin; Virus infection.
© Springer Nature Switzerland AG 2020.
Conflict of interest statement
Conflict of InterestThe authors declare that they have no conflict of interest. The Rajsbaum lab is supported by grants R01AI134907, R21AI126012, and R21AI132479 from the National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) and funds from UTMB Institute for Human Infections & Immunity (IHII). A.H. is supported by T32 AI007526 and S.v.T is supported by T32 AI060549 from NIH/NIAID.
Figures
Similar articles
-
The TRIMendous Role of TRIMs in Virus-Host Interactions.Vaccines (Basel). 2017 Aug 22;5(3):23. doi: 10.3390/vaccines5030023. Vaccines (Basel). 2017. PMID: 28829373 Free PMC article. Review.
-
To TRIM or not to TRIM: the balance of host-virus interactions mediated by the ubiquitin system.J Gen Virol. 2019 Dec;100(12):1641-1662. doi: 10.1099/jgv.0.001341. J Gen Virol. 2019. PMID: 31661051 Free PMC article.
-
The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication.J Virol. 2017 Aug 24;91(18):e00833-17. doi: 10.1128/JVI.00833-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28679761 Free PMC article.
-
The Dual Role of TRIM7 in Viral Infections.Viruses. 2024 Aug 12;16(8):1285. doi: 10.3390/v16081285. Viruses. 2024. PMID: 39205259 Free PMC article. Review.
-
The Roles of TRIMs in Antiviral Innate Immune Signaling.Front Cell Infect Microbiol. 2021 Mar 15;11:628275. doi: 10.3389/fcimb.2021.628275. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791238 Free PMC article. Review.
Cited by
-
Viral Evasion of RIG-I-Like Receptor-Mediated Immunity through Dysregulation of Ubiquitination and ISGylation.Viruses. 2021 Jan 26;13(2):182. doi: 10.3390/v13020182. Viruses. 2021. PMID: 33530371 Free PMC article. Review.
-
Cross-GWAS coherence test at the gene and pathway level.PLoS Comput Biol. 2022 Sep 26;18(9):e1010517. doi: 10.1371/journal.pcbi.1010517. eCollection 2022 Sep. PLoS Comput Biol. 2022. PMID: 36156592 Free PMC article.
-
Dual-Role Ubiquitination Regulation Shuttling the Entire Life Cycle of the Flaviviridae.Front Microbiol. 2022 May 6;13:835344. doi: 10.3389/fmicb.2022.835344. eCollection 2022. Front Microbiol. 2022. PMID: 35602051 Free PMC article. Review.
-
SARS-COV-2 protein NSP9 promotes cytokine production by targeting TBK1.Front Immunol. 2023 Oct 2;14:1211816. doi: 10.3389/fimmu.2023.1211816. eCollection 2023. Front Immunol. 2023. PMID: 37854611 Free PMC article.
-
The battle between host antiviral innate immunity and immune evasion by cytomegalovirus.Cell Mol Life Sci. 2024 Aug 9;81(1):341. doi: 10.1007/s00018-024-05369-y. Cell Mol Life Sci. 2024. PMID: 39120730 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
