The peroxisome counteracts oxidative stresses by suppressing catalase import via Pex14 phosphorylation
- PMID: 32831175
- PMCID: PMC7498260
- DOI: 10.7554/eLife.55896
The peroxisome counteracts oxidative stresses by suppressing catalase import via Pex14 phosphorylation
Abstract
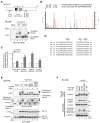
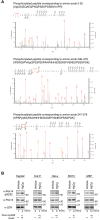
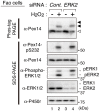
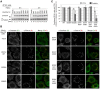
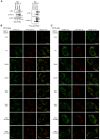
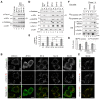
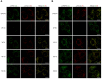
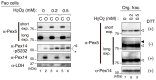
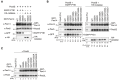
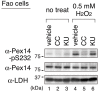
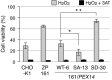
Most of peroxisomal matrix proteins including a hydrogen peroxide (H2O2)-decomposing enzyme, catalase, are imported in a peroxisome-targeting signal type-1 (PTS1)-dependent manner. However, little is known about regulation of the membrane-bound protein import machinery. Here, we report that Pex14, a central component of the protein translocation complex in peroxisomal membrane, is phosphorylated in response to oxidative stresses such as H2O2 in mammalian cells. The H2O2-induced phosphorylation of Pex14 at Ser232 suppresses peroxisomal import of catalase in vivo and selectively impairs in vitro the interaction of catalase with the Pex14-Pex5 complex. A phosphomimetic mutant Pex14-S232D elevates the level of cytosolic catalase, but not canonical PTS1-proteins, conferring higher cell resistance to H2O2. We thus suggest that the H2O2-induced phosphorylation of Pex14 spatiotemporally regulates peroxisomal import of catalase, functioning in counteracting action against oxidative stress by the increase of cytosolic catalase.
Keywords: Pex14; catalase; cell biology; human; hydrogen peroxide; oxidative stress; peroxisomal protein import; phosphorylation; rat.
© 2020, Okumoto et al.
Conflict of interest statement
KO, MN, HK, RN, TM, YF No competing interests declared, ME Mahmoud El Shermely is affiliated with Basilea Pharmaceutica International Ltd. The author has no financial interests to declare.
Figures



Similar articles
-
The peroxisomal import receptor PEX5 functions as a stress sensor, retaining catalase in the cytosol in times of oxidative stress.Biochim Biophys Acta Mol Cell Res. 2017 Oct;1864(10):1833-1843. doi: 10.1016/j.bbamcr.2017.07.013. Epub 2017 Jul 29. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28760655
-
A novel Pex14 protein-interacting site of human Pex5 is critical for matrix protein import into peroxisomes.J Biol Chem. 2014 Jan 3;289(1):437-48. doi: 10.1074/jbc.M113.499707. Epub 2013 Nov 14. J Biol Chem. 2014. PMID: 24235149 Free PMC article.
-
PEX5 protein binds monomeric catalase blocking its tetramerization and releases it upon binding the N-terminal domain of PEX14.J Biol Chem. 2011 Nov 25;286(47):40509-19. doi: 10.1074/jbc.M111.287201. Epub 2011 Oct 5. J Biol Chem. 2011. PMID: 21976670 Free PMC article.
-
Molecular insights into peroxisome homeostasis and peroxisome biogenesis disorders.Biochim Biophys Acta Mol Cell Res. 2022 Nov;1869(11):119330. doi: 10.1016/j.bbamcr.2022.119330. Epub 2022 Jul 30. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35917894 Review.
-
Cell Death or Survival Against Oxidative Stress.Subcell Biochem. 2018;89:463-471. doi: 10.1007/978-981-13-2233-4_20. Subcell Biochem. 2018. PMID: 30378036 Review.
Cited by
-
Peroxisomal PEX7 Receptor Affects Cadmium-Induced ROS and Auxin Homeostasis in Arabidopsis Root System.Antioxidants (Basel). 2021 Sep 20;10(9):1494. doi: 10.3390/antiox10091494. Antioxidants (Basel). 2021. PMID: 34573126 Free PMC article.
-
Peroxisome Deficiency in Cochlear Hair Cells Causes Hearing Loss by Deregulating BK Channels.Adv Sci (Weinh). 2023 Jul;10(20):e2300402. doi: 10.1002/advs.202300402. Epub 2023 May 12. Adv Sci (Weinh). 2023. PMID: 37171794 Free PMC article.
-
Mitigation of oxidative stress and inflammatory factors, along with the antibrowning and antimicrobial effects of cassia seed microbial fermentation solution.Front Microbiol. 2024 May 9;15:1400505. doi: 10.3389/fmicb.2024.1400505. eCollection 2024. Front Microbiol. 2024. PMID: 38784817 Free PMC article.
-
Current advances in the function and biogenesis of peroxisomes and their roles in health and disease.Histochem Cell Biol. 2021 Apr;155(4):513-524. doi: 10.1007/s00418-021-01982-1. Epub 2021 Apr 5. Histochem Cell Biol. 2021. PMID: 33818645 Free PMC article. No abstract available.
-
Mammalian pexophagy at a glance.J Cell Sci. 2024 May 1;137(9):jcs259775. doi: 10.1242/jcs.259775. Epub 2024 May 16. J Cell Sci. 2024. PMID: 38752931 Free PMC article. Review.
References
-
- Abe Y, Honsho M, Itoh R, Kawaguchi R, Fujitani M, Fujiwara K, Hirokane M, Matsuzaki T, Nakayama K, Ohgi R, Marutani T, Nakayama KI, Yamashita T, Fujiki Y. Peroxisome biogenesis deficiency attenuates the BDNF-TrkB pathway-mediated development of the cerebellum. Life Science Alliance. 2018;1:e201800062. doi: 10.26508/lsa.201800062. - DOI - PMC - PubMed
-
- Abe Y, Honsho M, Kawaguchi R, Matsuzaki T, Ichiki Y, Fujitani M, Fujiwara K, Hirokane M, Oku M, Sakai Y, Yamashita T, Fujiki Y. A peroxisome deficiency-induced reductive cytosol state up-regulates the brain-derived neurotrophic factor pathway. Journal of Biological Chemistry. 2020;295:5321–5334. doi: 10.1074/jbc.RA119.011989. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- JP26116007/MEXT/International
- JP24770130/Japan Society for the Promotion of Science/International
- JP26440032/Japan Society for the Promotion of Science/International
- JP17K07310/Japan Society for the Promotion of Science/International
- JP25112518/Japan Society for the Promotion of Science/International
- JP24247038/Japan Society for the Promotion of Science/International
- JP25116717/Japan Society for the Promotion of Science/International
- JP15K14511/Japan Society for the Promotion of Science/International
- JP15K21743/Japan Society for the Promotion of Science/International
- JP17H03675/Japan Society for the Promotion of Science/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

