Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines
- PMID: 32830112
- PMCID: PMC7445348
- DOI: 10.1136/jitc-2020-000873
Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines
Abstract
Background: Although programmed cell death-1/programmed death-ligand 1 (PD-L1) inhibitors show remarkable antitumor activity, a large portion of patients with cancer, even those with high PD-L1-expressing tumors, do not respond to their effects. Most PD-L1 inhibitors contain modified fragment crystallizable region (Fc) receptor binding sites to prevent antibody-dependent cellular cytotoxicity (ADCC) against PD-L1-expressing non-tumor cells. However, natural killer (NK) cells have specific antitumor activity in the presence of tumor-targeting antibody through ADCC, which could enhance NK cell-induced cytotoxicity. We evaluated the antitumor efficacy of ADCC via anti-PD-L1 monoclonal antibodies (mAbs) and NK cells against several PD-L1-positive cancer cell lines.
Methods: Various cancer cell lines were used as target cell lines. Surface PD-L1 expression was analyzed by flow cytometry. IMC-001 and anti-hPD-L1-hIgG1 were tested as anti-PD-L1 mAbs with ADCC and atezolizumab as an anti-PD-L1 mAb without ADCC. NK cell cytotoxicity was measured by 51Cr-release assay and CD107a degranulation assay. Also, live cell imaging was performed to evaluate cytotoxicity in a single-cell level. NK-92-CD16 (CD16-transduced NK-92 cell line) and peripheral blood mononuclear cells from healthy donors, respectively, were used as an effector cell. FcγRIIIa (CD16a)-V158F genotyping was performed for healthy donors.
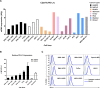
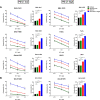
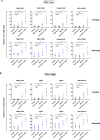
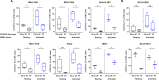
Results: We demonstrated that the cytotoxicity of NK-92-CD16 cells toward PD-L1-positive cancer cell lines was significantly enhanced in the presence of anti-PD-L1 mAb with ADCC. We also noted a significant increase in primary human NK cell cytotoxicity against PD-L1-positive human cancer cells when cocultured with anti-PD-L1 mAb with ADCC. Moreover, NK cells expressing a FCGR3A high-affinity genotype displayed higher anti-PD-L1 mAb-mediated ADCC lysis of tumor cells than donors with a low-affinity genotype.
Conclusion: These results suggest that NK cells induce an ADCC response in combination with anti-PD-L1 mAbs, which helps promote ADCC antitumor activity against PD-L1-positive tumors. This study provides support for NK cell immunotherapy against high PD-L1-expressing tumors in combination with ADCC through anti-PD-L1 mAbs.
Keywords: cytotoxicity; head and neck neoplasms; immunological; immunotherapy; killer cells; lung neoplasms; natural.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells.Cancer Immunol Res. 2015 Oct;3(10):1148-1157. doi: 10.1158/2326-6066.CIR-15-0059. Epub 2015 May 26. Cancer Immunol Res. 2015. PMID: 26014098 Free PMC article.
-
Avelumab, an IgG1 anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production Against Triple Negative Breast Cancer Cells.Front Immunol. 2018 Sep 20;9:2140. doi: 10.3389/fimmu.2018.02140. eCollection 2018. Front Immunol. 2018. PMID: 30294328 Free PMC article.
-
A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab.J Neurosurg. 2018 May;128(5):1419-1427. doi: 10.3171/2017.1.JNS162610. Epub 2017 Jul 28. J Neurosurg. 2018. PMID: 28753113 Free PMC article.
-
Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells.Cancers (Basel). 2021 Jan 16;13(2):312. doi: 10.3390/cancers13020312. Cancers (Basel). 2021. PMID: 33467027 Free PMC article. Review.
-
Potentiation of natural killer cells to overcome cancer resistance to NK cell-based therapy and to enhance antibody-based immunotherapy.Front Immunol. 2023 Nov 24;14:1275904. doi: 10.3389/fimmu.2023.1275904. eCollection 2023. Front Immunol. 2023. PMID: 38077389 Free PMC article. Review.
Cited by
-
Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors.J Immunother Cancer. 2024 Feb 2;12(2):e007340. doi: 10.1136/jitc-2023-007340. J Immunother Cancer. 2024. PMID: 38309722 Free PMC article. Clinical Trial.
-
ISG12a promotes immunotherapy of HBV-associated hepatocellular carcinoma through blocking TRIM21/AKT/β-catenin/PD-L1 axis.iScience. 2024 Mar 19;27(4):109533. doi: 10.1016/j.isci.2024.109533. eCollection 2024 Apr 19. iScience. 2024. PMID: 38591006 Free PMC article.
-
Unlocking Benzosampangine's Potential: A Computational Approach to Investigating, Its Role as a PD-L1 Inhibitor in Tumor Immune Evasion via Molecular Docking, Dynamic Simulation, and ADMET Profiling.Bioinform Biol Insights. 2024 Nov 18;18:11779322241298591. doi: 10.1177/11779322241298591. eCollection 2024. Bioinform Biol Insights. 2024. PMID: 39564188 Free PMC article.
-
Innate Lymphoid Cells in Bladder Cancer: From Mechanisms of Action to Immune Therapies.Cancer Immunol Res. 2024 Feb 2;12(2):149-160. doi: 10.1158/2326-6066.CIR-23-0414. Cancer Immunol Res. 2024. PMID: 38060011 Free PMC article. Review.
-
Oxidative stress in the tumor microenvironment in gastric cancer and its potential role in immunotherapy.FEBS Open Bio. 2023 Jul;13(7):1238-1252. doi: 10.1002/2211-5463.13630. Epub 2023 May 20. FEBS Open Bio. 2023. PMID: 37171226 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
