NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice-A Novel in Vivo Imaging Platform
- PMID: 32824188
- PMCID: PMC7461499
- DOI: 10.3390/ijms21165863
NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice-A Novel in Vivo Imaging Platform
Abstract
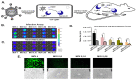
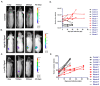
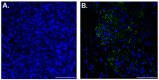
Reporter genes are used to visualize intracellular biological phenomena, including viral infection. Here we demonstrate bioluminescent imaging of viral infection using the NanoBiT system in combination with intraperitoneal injection of a furimazine analogue, hydrofurimazine. This recently developed substrate has enhanced aqueous solubility allowing delivery of higher doses for in vivo imaging. The small high-affinity peptide tag (HiBiT), which is only 11 amino-acids in length, was engineered into a clinically used oncolytic adenovirus, and the complementary large protein (LgBiT) was constitutively expressed in tumor cells. Infection of the LgBiT expressing cells with the HiBiT oncolytic virus will reconstitute NanoLuc in the cytosol of the cell, providing strong bioluminescence upon treatment with substrate. This new bioluminescent system served as an early stage quantitative viral transduction reporter in vitro and also in vivo in mice, for longitudinal monitoring of oncolytic viral persistence in infected tumor cells. This platform provides novel opportunities for studying the biology of viruses in animal models.
Keywords: bioluminescence imaging; hibit tag; hydrofurimazine; nanobit system; oncolytic virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of NanoLuc substrates for bioluminescence imaging of transferred cells in mice.J Photochem Photobiol B. 2021 Mar;216:112128. doi: 10.1016/j.jphotobiol.2021.112128. Epub 2021 Jan 26. J Photochem Photobiol B. 2021. PMID: 33529963
-
Fiat Luc: Bioluminescence Imaging Reveals In Vivo Viral Replication Dynamics.PLoS Pathog. 2015 Sep 10;11(9):e1005081. doi: 10.1371/journal.ppat.1005081. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26356297 Free PMC article. No abstract available.
-
Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals.Nat Methods. 2020 Aug;17(8):852-860. doi: 10.1038/s41592-020-0889-6. Epub 2020 Jul 13. Nat Methods. 2020. PMID: 32661427 Free PMC article.
-
In Vivo Live Imaging of Oncolytic Mammalian Orthoreovirus Expressing NanoLuc Luciferase in Tumor Xenograft Mice.J Virol. 2019 Jun 28;93(14):e00401-19. doi: 10.1128/JVI.00401-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31068423 Free PMC article.
-
CRISPR-Mediated Tagging of Endogenous Proteins with a Luminescent Peptide.ACS Chem Biol. 2018 Feb 16;13(2):467-474. doi: 10.1021/acschembio.7b00549. Epub 2017 Sep 21. ACS Chem Biol. 2018. PMID: 28892606
Cited by
-
Detecting SARS-CoV-2 neutralizing immunity: highlighting the potential of split nanoluciferase technology.J Mol Cell Biol. 2022 Aug 17;14(4):mjac023. doi: 10.1093/jmcb/mjac023. J Mol Cell Biol. 2022. PMID: 35416249 Free PMC article. Review.
-
Integration of HiBiT into enteroviruses: A universal tool for advancing enterovirus virology research.Virol Sin. 2024 Jun;39(3):422-433. doi: 10.1016/j.virs.2024.03.004. Epub 2024 Mar 16. Virol Sin. 2024. PMID: 38499155 Free PMC article.
-
Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase.Cell Commun Signal. 2023 Jun 14;21(1):136. doi: 10.1186/s12964-023-01146-9. Cell Commun Signal. 2023. PMID: 37316874 Free PMC article.
-
Development of a HiBiT-tagged reporter hepatitis E virus and its utility as an antiviral drug screening platform.J Virol. 2023 Sep 28;97(9):e0050823. doi: 10.1128/jvi.00508-23. Epub 2023 Sep 8. J Virol. 2023. PMID: 37681960 Free PMC article.
-
Intracellular Ionic Strength Sensing Using NanoLuc.Int J Mol Sci. 2021 Jan 12;22(2):677. doi: 10.3390/ijms22020677. Int J Mol Sci. 2021. PMID: 33445497 Free PMC article.
References
-
- Schoggins J.W., Dorner M., Feulner M., Imanaka N., Murphy M.Y., Ploss A., Rice C.M. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA. 2012;109:14610. doi: 10.1073/pnas.1212379109. - DOI - PMC - PubMed
-
- Luker G.D., Leib D.A. Luciferase real-time bioluminescence imaging for the study of viral pathogenesis. Methods Mol. Biol. 2005;292:285–296. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

