Early Intervention in Ulcerative Colitis: Ready for Prime Time?
- PMID: 32823997
- PMCID: PMC7464940
- DOI: 10.3390/jcm9082646
Early Intervention in Ulcerative Colitis: Ready for Prime Time?
Abstract
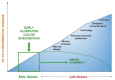
Growing evidence shows that ulcerative colitis (UC) is a progressive disease similar to Crohn's disease (CD). The UC-related burden is often underestimated by physicians and a standard step-up therapeutic approach is preferred. However, in many patients with UC the disease activity is not adequately controlled by current management, leading to poor long-term prognosis. Data from both randomized controlled trials and real-world studies support early intervention in CD in order to prevent disease progression and irreversible bowel damage. Similarly, an early disease intervention during the so-called "window of opportunity" could lead to better outcomes in UC. Here, we summarize the literature evidence on early intervention in patients with UC, highlighting strengths and limitations of this approach.
Keywords: early intervention; top-down strategy; treat-to-target; ulcerative colitis; window of opportunity.
Conflict of interest statement
V.S., F.D. and E.Z. declare no conflict of interest. L.P.-B. has served as a speaker, consultant and advisory board member for Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC Pharma, Index Pharmaceuticals, Amgen, Sandoz, For- ward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, Theravance. S.D. has served as a speaker, consultant and advisory board member for Schering-Plough, AbbVie, MSD, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alphawasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor and Johnson & Johnson, Nikkiso Europe GMBH, Theravance.
Figures
Similar articles
-
Colorectal adenocarcinoma in Crohn's disease: a retrospective histologic study.Dis Colon Rectum. 1997 Sep;40(9):1072-8. doi: 10.1007/BF02050932. Dis Colon Rectum. 1997. PMID: 9293938
-
Generalized Pyoderma Gangrenosum Associated with Ulcerative Colitis: Successful Treatment with Infliximab and Azathioprine.Acta Dermatovenerol Croat. 2016 Apr;24(1):83-5. Acta Dermatovenerol Croat. 2016. PMID: 27149138
-
Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America.J Pediatr Gastroenterol Nutr. 2007 May;44(5):653-74. doi: 10.1097/MPG.0b013e31805563f3. J Pediatr Gastroenterol Nutr. 2007. PMID: 17460505
-
Long-term safety of adalimumab in clinical trials in adult patients with Crohn's disease or ulcerative colitis.Aliment Pharmacol Ther. 2018 Jan;47(2):219-228. doi: 10.1111/apt.14420. Epub 2017 Nov 21. Aliment Pharmacol Ther. 2018. PMID: 29159817
-
A review of infliximab use in ulcerative colitis.Clin Ther. 2008 Feb;30(2):223-30. doi: 10.1016/j.clinthera.2008.02.014. Clin Ther. 2008. PMID: 18343261 Review.
Cited by
-
Change in systemic steroid use and surgery rate in patients with inflammatory bowel disease: a Japanese real-world database analysis.J Gastroenterol. 2024 May;59(5):389-401. doi: 10.1007/s00535-024-02086-y. Epub 2024 Mar 16. J Gastroenterol. 2024. PMID: 38492011 Free PMC article.
-
The Guide to Guidelines in Ulcerative Colitis: Interpretation and Appropriate Use in Clinical Practice.Gastroenterol Hepatol (N Y). 2021 Apr;17(4 Suppl 4):3-13. Gastroenterol Hepatol (N Y). 2021. PMID: 34135718 Free PMC article. No abstract available.
-
A scoping review on early inflammatory bowel disease: definitions, pathogenesis, and impact on clinical outcomes.Therap Adv Gastroenterol. 2022 Dec 19;15:17562848221142673. doi: 10.1177/17562848221142673. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36569381 Free PMC article.
-
Risk minimization of JAK inhibitors in ulcerative colitis following regulatory guidance.Nat Rev Gastroenterol Hepatol. 2023 Mar;20(3):129-130. doi: 10.1038/s41575-022-00722-7. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36536208 No abstract available.
-
Biomarker discovery for personalized therapy selection in inflammatory bowel diseases: Challenges and promises.Curr Res Pharmacol Drug Discov. 2022 Jan 26;3:100089. doi: 10.1016/j.crphar.2022.100089. eCollection 2022. Curr Res Pharmacol Drug Discov. 2022. PMID: 35146421 Free PMC article. Review.
References
-
- Torres J., Billioud V., Sachar D.B., Peyrin-Biroulet L., Colombel J.F. Ulcerative colitis as a progressive disease: The forgotten evidence. Inflamm. Bowel Dis. 2012;18:1356–1363. - PubMed
-
- Peyrin-Biroulet L., Sandborn W., Sands B.E., Reinisch W., Bemelman W., Bryant R.V., D’Haens G., Dotan I., Dubinsky M., Feagan B., et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015;110:1324–1338. - PubMed
-
- Peyrin-Biroulet L., Bressenot A., Kampman W. Histologic Remission: The Ultimate Therapeutic Goal in Ulcerative Colitis? Clin. Gastroenterol. Hepatol. 2014;12:929–934.e2. - PubMed
-
- Danese S., Fiorino G., Peyrin-Biroulet L. Early intervention in Crohn’s disease: Towards disease modification trials. Gut. 2017;66:2179–2187. - PubMed
Publication types
LinkOut - more resources
Full Text Sources