Talin folding as the tuning fork of cellular mechanotransduction
- PMID: 32817549
- PMCID: PMC7474635
- DOI: 10.1073/pnas.2004091117
Talin folding as the tuning fork of cellular mechanotransduction
Abstract
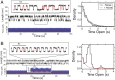
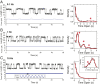
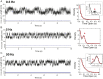
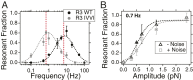
Cells continually sample their mechanical environment using exquisite force sensors such as talin, whose folding status triggers mechanotransduction pathways by recruiting binding partners. Mechanical signals in biology change quickly over time and are often embedded in noise; however, the mechanics of force-sensing proteins have only been tested using simple force protocols, such as constant or ramped forces. Here, using our magnetic tape head tweezers design, we measure the folding dynamics of single talin proteins in response to external mechanical noise and cyclic force perturbations. Our experiments demonstrate that talin filters out external mechanical noise but detects periodic force signals over a finely tuned frequency range. Hence, talin operates as a mechanical band-pass filter, able to read and interpret frequency-dependent mechanical information through its folding dynamics. We describe our observations in the context of stochastic resonance, which we propose as a mechanism by which mechanosensing proteins could respond accurately to force signals in the naturally noisy biological environment.
Keywords: magnetic tweezers; protein folding; stochastic resonance; talin.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
All Subdomains of the Talin Rod Are Mechanically Vulnerable and May Contribute To Cellular Mechanosensing.ACS Nano. 2016 Jul 26;10(7):6648-58. doi: 10.1021/acsnano.6b01658. Epub 2016 Jul 11. ACS Nano. 2016. PMID: 27380548 Free PMC article.
-
Uncovering mechanosensing mechanisms at the single protein level using magnetic tweezers.Methods. 2016 Feb 1;94:13-8. doi: 10.1016/j.ymeth.2015.08.020. Epub 2015 Aug 28. Methods. 2016. PMID: 26318089 Review.
-
The mechanical response of talin.Nat Commun. 2016 Jul 7;7:11966. doi: 10.1038/ncomms11966. Nat Commun. 2016. PMID: 27384267 Free PMC article.
-
Direct single-molecule quantification reveals unexpectedly high mechanical stability of vinculin-talin/α-catenin linkages.Sci Adv. 2019 Dec 18;5(12):eaav2720. doi: 10.1126/sciadv.aav2720. eCollection 2019 Dec. Sci Adv. 2019. PMID: 31897422 Free PMC article.
-
Talin Dependent Mechanosensitivity of Cell Focal Adhesions.Cell Mol Bioeng. 2015;8(1):151-159. doi: 10.1007/s12195-014-0364-5. Epub 2014 Nov 4. Cell Mol Bioeng. 2015. PMID: 26097520 Free PMC article. Review.
Cited by
-
Interdomain Linker Effect on the Mechanical Stability of Ig Domains in Titin.Int J Mol Sci. 2022 Aug 30;23(17):9836. doi: 10.3390/ijms23179836. Int J Mol Sci. 2022. PMID: 36077234 Free PMC article.
-
Protein nanomechanics in biological context.Biophys Rev. 2021 Aug 7;13(4):435-454. doi: 10.1007/s12551-021-00822-9. eCollection 2021 Aug. Biophys Rev. 2021. PMID: 34466164 Free PMC article. Review.
-
Durotaxis: the mechanical control of directed cell migration.FEBS J. 2022 May;289(10):2736-2754. doi: 10.1111/febs.15862. Epub 2021 May 7. FEBS J. 2022. PMID: 33811732 Free PMC article. Review.
-
The role of single protein elasticity in mechanobiology.Nat Rev Mater. 2023 Jan;8:10-24. doi: 10.1038/s41578-022-00488-z. Epub 2022 Oct 24. Nat Rev Mater. 2023. PMID: 37469679 Free PMC article.
-
Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells.Cells. 2024 Jan 2;13(1):96. doi: 10.3390/cells13010096. Cells. 2024. PMID: 38201302 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

